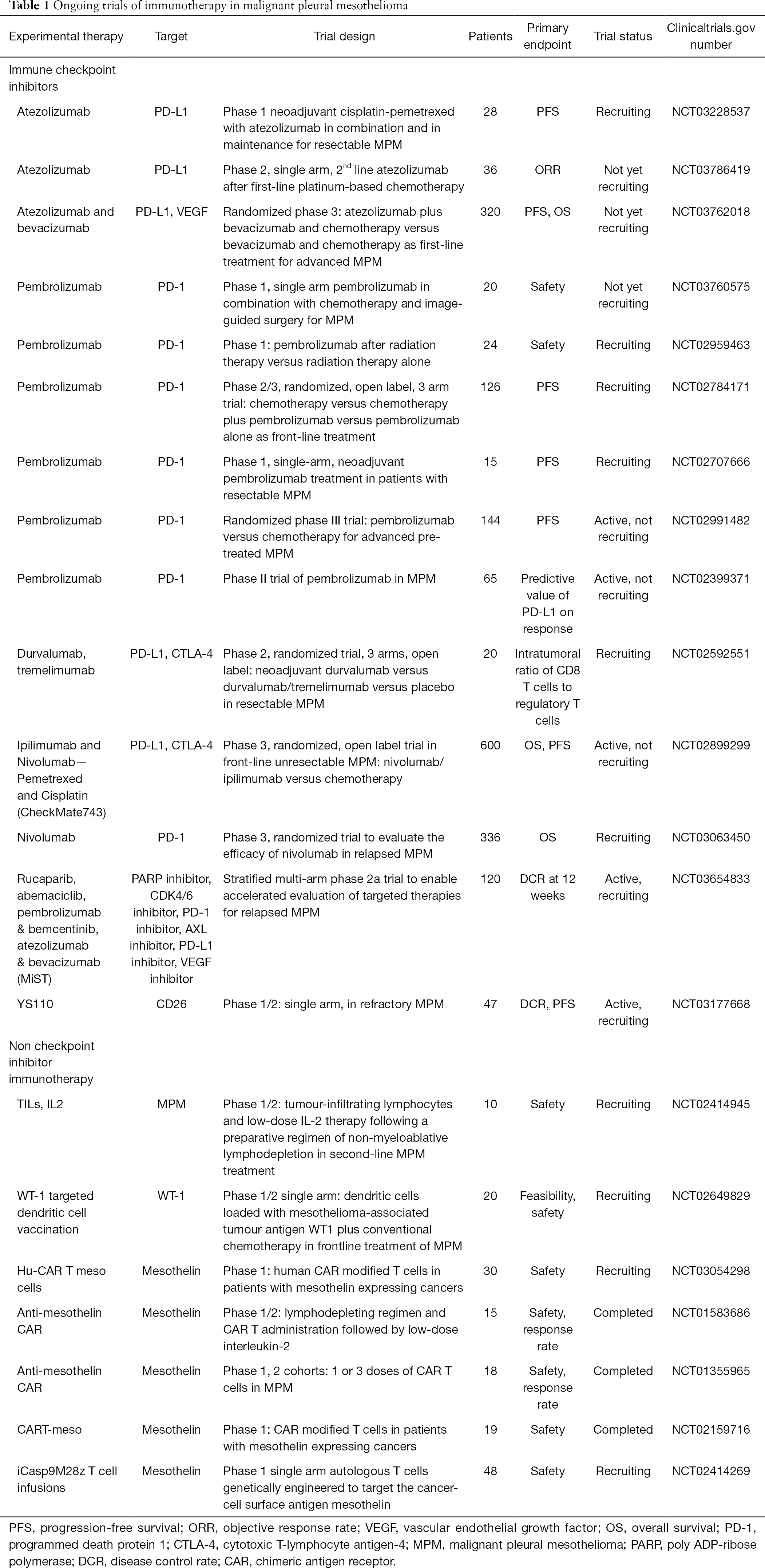
Nivolumab Mesothelioma Uk, Http Www Io Nihr Ac Uk Wp Content Uploads Migrated New 13775 Opdivo With Platinum Double Chemotherapy For Nsclc Pdf
Nivolumab mesothelioma uk Indeed lately has been hunted by users around us, maybe one of you personally. People are now accustomed to using the net in gadgets to see video and image information for inspiration, and according to the name of this article I will talk about about Nivolumab Mesothelioma Uk.
- Wish Lab Southampton On Twitter Confirm Immunotherapy Phase Iii Trial Looking At Nivolumab Anti Pd1 Receptor In Relapsed Mesothelioma Poster Uos Medicine Faculty Conference Today Southamptonctu Cruksouthampton Rdsouthampton Https T Co
- Malignant Pleural Mesothelioma Ppt Download
- First Line Nivolumab Ipilimumab Combination Improves Os In Mesothelioma Subset
- New Immune Checkpoint Inhibitor Drugs Approved For Mesothelioma
- Anti Angiogenic And Immunotherapeutic Approaches In Mesothelioma Memoinoncology
- Nivolumab Or Nivolumab Plus Ipilimumab In Patients With Relapsed Malignant Pleural Mesothelioma Ifct 1501 Maps2 A Multicentre Open Label Randomised Non Comparative Phase 2 Trial Sciencedirect
Find, Read, And Discover Nivolumab Mesothelioma Uk, Such Us:
- Anti Angiogenic And Immunotherapeutic Approaches In Mesothelioma Memoinoncology
- Physician With Mesothelioma Wins Legal Case Against Uk S National Health Service Mesothelioma Net
- Fda Approves Bms Opdivo And Yervoy Combination In First Line Mesothelioma
- Nivolumab Or Nivolumab Plus Ipilimumab In Patients With Relapsed Malignant Pleural Mesothelioma Ifct 1501 Maps2 A Multicentre Open Label Randomised Non Comparative Phase 2 Trial The Lancet Oncology
- Mesothelioma Uk Posts Facebook
- Normal Lung Cells Under Microscope
- Mesothelioma Right Parietal Pleura Icd O 3
- Timeshare Lawyers
- Mesothelioma Rates Uk
- Is Mesothelioma Cancer Genetic
If you re looking for Is Mesothelioma Cancer Genetic you've come to the perfect place. We have 100 graphics about is mesothelioma cancer genetic adding pictures, photos, pictures, wallpapers, and more. In these page, we additionally provide variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.

Nivolumab Mesothelioma Clinical Trials Search For Answers Mesothelioma Circle Is Mesothelioma Cancer Genetic

Phase 3 Clinical Trial To Test Opdivo In Mesothelioma Patients In United Kingdom The Asbestos Cancer Organization Is Mesothelioma Cancer Genetic
Researchers want to find out if nivolumab can help these people.

Is mesothelioma cancer genetic. Nivolumab a life extending immunotherapy drug used for lung cancers approved for nhs use. Nivolumab is a type of immunotherapy drug called a monoclonal antibodyit works by stimulating the bodys immune system to recognise and kill cancer cells. Common side effects include tiredness rash liver problems muscles pains and cough.
Nivolumab a life extending immunotherapy drug will be made immediately available to nhs patients across england suffering from certain lung cancers which may include some mesothelioma sufferers. There is a need to evaluate checkpoint inhibitors in patients with relapsed mesothelioma where treatment options are limited. Here we assessed the safety and efficacy of the combination of nivolumab an anti programmed cell death 1 antibody plus ipilimumab an anti cytotoxic t lymphocyte protein 4 antibody in patients with previously treated and relapsed malignant pleural mesothelioma.
Doctors treat mesothelioma with chemotherapyafter chemotherapy if the mesothelioma comes back the aim is to control symptoms. By next fall researchers anticipate to collect more data from the maps 2 trial including more information about mature survival quality of life and biomarker data. Mesothelioma option to give nivolumab monotherapy instead of second line chemotherapy to reduce risk of immunosuppression myeloma option to give oral pomalidomide with dexamethasone as second or third line therapy instead of intravenous treatments in patients previously treated with lenalidomide to reduce the.
The department for health and social care has asked nice to conduct an appraisal of nivolumab with ipilimumab for untreated unresectable malignant pleural mesothelioma. Please note that following on from advice received from the company the timelines for this appraisal have been revised. A company limited by guarantee.
The addition of 12 months of nivolumab anti pd1 antibody to standard practice will be conducted in the uk using a randomised placebo controlled phase iii trial the cancer research uk confirm trial. This includes melanoma lung cancer renal cell carcinoma hodgkin lymphoma head and neck cancer colon cancer and liver cancer. 21st sep 17 229 pm.
Single drug checkpoint inhibition has shown efficacy in patients with recurrent malignant pleural mesothelioma. Cancer research uk is a registered charity in england and wales 1089464 scotland sc041666 the isle of man 1103 and jersey 247. These regimens require confirmation in larger clinical trials.
Anti pd 1 nivolumab monotherapy or nivolumab plus anti ctla 4 ipilimumab combination therapy both showed promising activity in relapsed patients with malignant pleural mesothelioma without unexpected toxicity. Nivolumab sold under the brand name opdivo is a medication used to treat a number of types of cancer. This is called active symptom control.
More From Is Mesothelioma Cancer Genetic
- Simmons Conroy
- Jacoby Miles Today
- Victoria John Mayer
- Neil Patrick Harris Halloween
- Mickey Mouse Colouring Pages
Incoming Search Terms:
- Mesothelioma Uk Posts Facebook Mickey Mouse Colouring Pages,
- Malignant Pleural Mesothelioma Ppt Download Mickey Mouse Colouring Pages,
- The Role Of Immunotherapy In Mesothelioma Colarusso Precision Cancer Medicine Mickey Mouse Colouring Pages,
- Side Effects Of Opdivo Can Be Deadly For Some Mesothelioma Patients Mickey Mouse Colouring Pages,
- Nivolumab New Immunotherapy Drug Could Treat Mesothelioma The Cancer Caused By Asbestos Mickey Mouse Colouring Pages,
- 0i8fmudtohs0km Mickey Mouse Colouring Pages,