Nintedanib Mesothelioma Fda, Ofev Nintedanib Dosing Indications Interactions Adverse Effects And More
Nintedanib mesothelioma fda Indeed recently is being sought by users around us, maybe one of you personally. Individuals now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the title of the article I will discuss about Nintedanib Mesothelioma Fda.
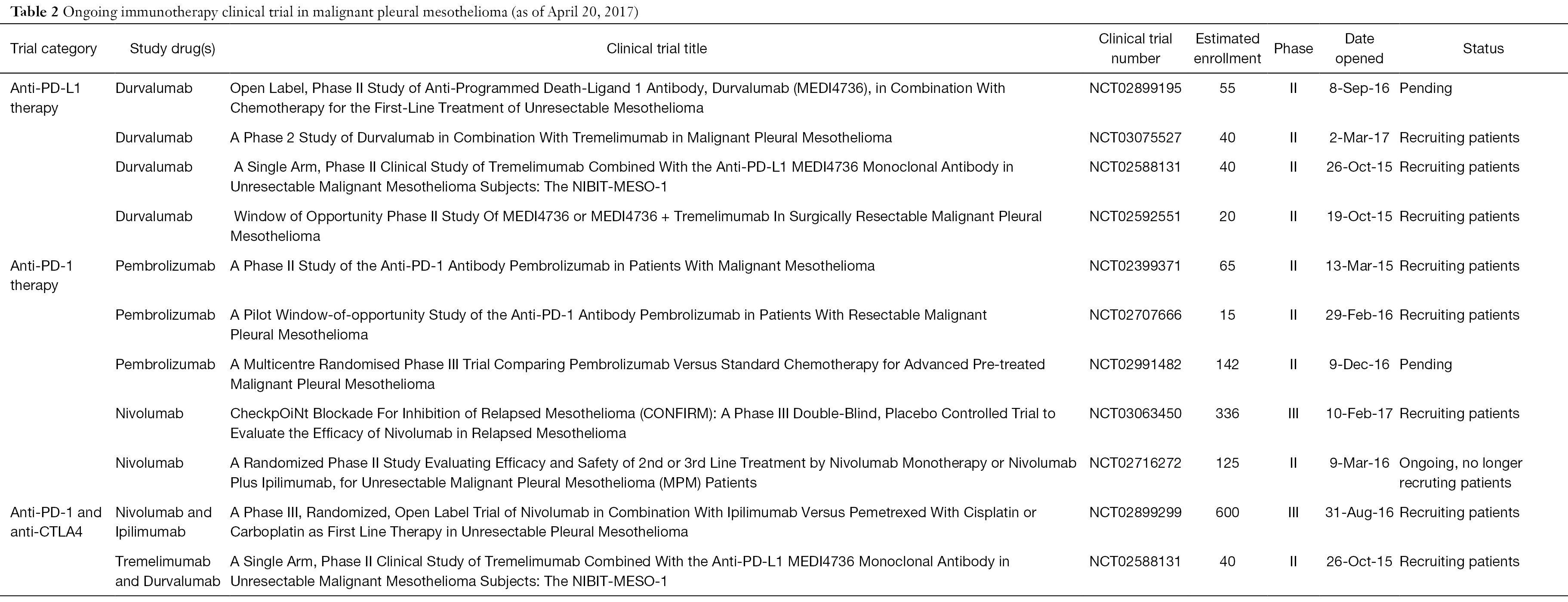
- Searching For Targets For The Systemic Therapy Of Mesothelioma Annals Of Oncology
- Full Text Analysis Of Patient Preferences In Lung Cancer Estimating Acce Ppa
- Nintedanib In Combination With Pemetrexed And Cisplatin For Chemotherapy Naive Patients With Advanced Malignant Pleural Mesothelioma Lume Meso A Double Blind Randomised Placebo Controlled Phase 3 Trial Sciencedirect
- Boehringer Mulai Mencoba Efikasi Dan Keamanan Nintedanib Untuk Pleural Mesothelioma Info Farmasi Terkini Berbasis Ilmiah Dan Praktis
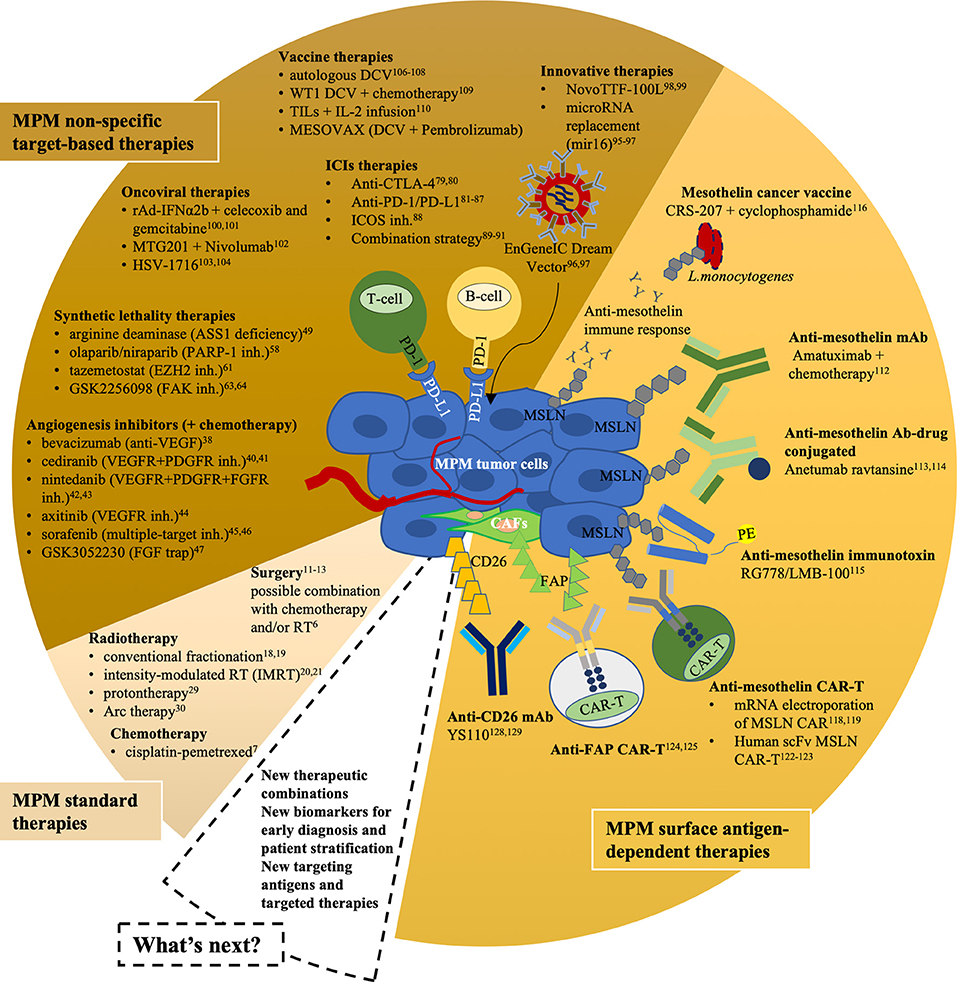
- New Therapeutic Strategies For Malignant Pleural Mesothelioma Sciencedirect
- Https Www Fda Gov Media 129747 Download
Find, Read, And Discover Nintedanib Mesothelioma Fda, Such Us:
- Nintedanib Esylate Bibf 1120 Esylate Pdgfr Vegfr Fgfr Inhibitor Medchemexpress
- Mesothelioma Research Diagnostic Tools Treatments Information
- Searching For Targets For The Systemic Therapy Of Mesothelioma Annals Of Oncology
- Current Chemotherapy Strategies In Malignant Pleural Mesothelioma De Gooijer Translational Lung Cancer Research
- New York City Truck Accident Lawyer
- Reasonable Divorce Lawyers
- Roblox Coloring Pages To Print
- Letter W Coloring Pages Preschool
- Treatment Mesothelioma Lung
If you re searching for Treatment Mesothelioma Lung you've come to the perfect location. We have 100 graphics about treatment mesothelioma lung including pictures, photos, pictures, wallpapers, and more. In such page, we also have variety of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.

Full Text Spotlight On Bevacizumab And Its Potential In The Treatment Of Maligna Ott Treatment Mesothelioma Lung
Boehringer ingelheim pharmaceuticals inc.

Treatment mesothelioma lung. Food and drug administration fda in december 2016 granted nintedanib an orphan drug designation for mesothelioma encouraging its use before officially approving it. Orphan drug designation is granted by the fda to investigational compounds intended for the safe and effective treatment diagnosis or prevention of rare diseases or disorders that. And some have a dummy drug placebo.
Nintedanib is a type of targeted drug called a cancer growth blocker. Ofev fda approval history. Reduced mpm cell proliferation and migration and the inhibition of erk12 phosphorylation were also observed upon nintedanib treatment in vitro additive effects on cell viability were detected when nintedanib was combined with cisplatin a drug routinely used.
Ridgefield conn december 14 2016 boehringer ingelheim today announced that the us. Nintedanib bibf 1120 in mesothelioma the safety and scientific validity of this study is the responsibility of the study sponsor and investigators. It stops signals that cancer cells use to divide and grow.
Mhra and the fda are moving the drug another step closer to changing the long accepted but relatively ineffective standard of care treatment for unresectable pleural. The main aims of the trial are to. The angiokinase inhibitor nintedanib has shown promising activity in the lume meso phase ii mpm trial and thus is currently being evaluated in the confirmatory lume meso phase iii trial.
Yes first approved october 15 2014 brand name. Nintedanib is currently being investigated in patients with various solid tumours including phase iii studies in advanced nsclc1 1 colorectal cancer refractory to standard treatment 6 and ovarian cancer 7 and also in phase ii studies in mesothelioma 8 kidney cancer renal cell carcinoma 9 and liver cancer hepatic cell carcinoma 10. In this trial some people have nintedanib.
Treatment of idiopathic pulmonary fibrosis. Nintedanib is marketed under the brand name ofev. Interstitial lung disease ofev nintedanib is a small molecule tyrosine kinase inhibitor tki indicated for.
Food and drug administration fda has granted orphan drug designation to nintedanib for the treatment of mesothelioma. Listing a study does not mean it has been evaluated by the us. Listing a study does not mean it has been evaluated by the us.
Nintedanib inhibited mpm cell growth in both short and long term viability assays. Nintedanib shows promise as an addition to the standard chemotherapy regimen for pleural mesothelioma. Nintedanib bibf 1120 in mesothelioma the safety and scientific validity of this study is the responsibility of the study sponsor and investigators.

Nintedanib In Combination With Pemetrexed And Cisplatin For Chemotherapy Naive Patients With Advanced Malignant Pleural Mesothelioma Lume Meso A Double Blind Randomised Placebo Controlled Phase 3 Trial The Lancet Respiratory Medicine Treatment Mesothelioma Lung
More From Treatment Mesothelioma Lung
- Sofia The First Coloring Pages Clover
- Law Office Of David Williams
- How Long Does It Take To Develop Mesothelioma
- John Mayer Married To Katy Perry
- Wwwasbestoscommesothelioma
Incoming Search Terms:
- Mesothelioma Medications For Chemotherapy Immunotherapy Wwwasbestoscommesothelioma,
- Frontiers Malignant Pleural Mesothelioma State Of The Art On Current Therapies And Promises For The Future Oncology Wwwasbestoscommesothelioma,
- Nintedanib Superior To Bevacizumab For Mesothelioma Treatment Wwwasbestoscommesothelioma,
- Lume Meso Trial In Malignant Pleural Mesothelioma Fails Endpoint Wwwasbestoscommesothelioma,
- Mesothelioma Treatment Are We On Target A Review Sciencedirect Wwwasbestoscommesothelioma,
- Https Www Fda Gov Media 129233 Download Wwwasbestoscommesothelioma,