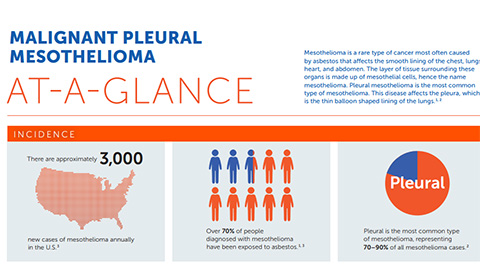
Mesothelioma Clinical Study, Ex 99 2
Mesothelioma clinical study Indeed recently is being hunted by consumers around us, maybe one of you. Individuals are now accustomed to using the net in gadgets to see image and video information for inspiration, and according to the title of this post I will discuss about Mesothelioma Clinical Study.
- Nintedanib In Combination With Pemetrexed And Cisplatin For Chemotherapy Naive Patients With Advanced Malignant Pleural Mesothelioma Lume Meso A Double Blind Randomised Placebo Controlled Phase 3 Trial The Lancet Respiratory Medicine
- Nivolumab Or Nivolumab Plus Ipilimumab In Patients With Relapsed Malignant Pleural Mesothelioma Ifct 1501 Maps2 A Multicentre Open Label Randomised Non Comparative Phase 2 Trial The Lancet Oncology
- 2
- Factors Associated With Survival In A Large Series Of Patients With Malignant Pleural Mesothelioma In New South Wales British Journal Of Cancer
- Why You Need To Enroll In A Mesothelioma Clinical Trial Mesothelioma Group
- New Gene Therapy Phase 3 Clinical Trial In Mesothelioma Mesothelioma Applied Research Foundation
Find, Read, And Discover Mesothelioma Clinical Study, Such Us:
- 13 18 Beat Meso Etop
- Clinical Trial Recruiting 30 More Pleural Mesothelioma Patients For Study
- Mesothelioma Treatment Surgery Chemotherapy Emerging Treatments
- Malignant Pleural Mesothelioma An Update On Investigation Diagnosis And Treatment European Respiratory Society
- Immunotherapy For Malignant Pleural Mesothelioma Current Status And Future Directions Dozier Translational Lung Cancer Research
- Newman Law Firm
- Pretty Mandala Coloring Pages
- Child Visitation Attorney
- Simmons Beautyrest Classic Firm
- Is Asbestos Dangerous If Not Disturbed
If you are looking for Is Asbestos Dangerous If Not Disturbed you've reached the perfect place. We ve got 100 graphics about is asbestos dangerous if not disturbed adding pictures, photos, photographs, wallpapers, and more. In such web page, we additionally have variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.

Mesothelioma Clinical Trials Improve Prognosis With New Treatments Is Asbestos Dangerous If Not Disturbed
Mesothelioma Clinical Trials Get Help Finding The Latest Treatments Is Asbestos Dangerous If Not Disturbed
Due to the availability of second line options either within the vim trial or off study the confirm trial aims to evaluate immunotherapy in the third line setting a clinical situation in which current standard.

Is asbestos dangerous if not disturbed. Memorial sloan kettering cancer center in new york city has begun recruiting patients with pleural mesothelioma for its latest and potentially most promising clinical trial involving t cell therapy. The aim of this study was to examine the prognostic implications of clinical and pathologic characteristics of malignant peritoneal mesothelioma in a large multiinstitutional cohort of patients. Early research has found a dependency of mesothelioma on the pd 1 checkpoint.
All trials on the list are supported by nci. Clinical trials look at new ways to prevent detect or treat disease. In part 1 12 subjects with relapsed or refractory malignant mesothelioma regardless of bap1 status will be treated and undergo pharmacokinetics pk blood sample collection after a single tazemetostat 800 mg.
A mesothelioma clinical trials consent form should include information about potential costs to participants and what is covered by the sponsor of the clinical trial. Food and drug administrations recent investigational drug application approval of ata2271 a chimeric antigen receptor known as car t cell therapy. This trial was designed in response to that survey.
Screening of subjects to determine eligibility for the study will be performed within 21 days of the first planned dose of tazemetostat. Purpose of the clinical study is to assess safety and effects of a cancer vaccine that is provided with nivolumab immunotherapy in patients who have pleural mesothelioma that continues to spread despite treatment. It uses the immunotherapy agent nivolumab which blocks programmed cell death 1 pd 1 receptor on activated t cells a type of white blood cell forming part of the immune system.
If a new treatment option is successful it may be considered for us. Nct02139904 trial is ongoing to evaluate its efficacy in this setting. Food and drug administration fda approval and can be readily available for patients outside of clinical trials.
Ncis basic information about clinical trials explains the types and phases of trials and how they are carried out. Patients can reach out to their doctor and cancer care team for help with the details of a particular trial and how to cover associated costs. The novel study stems from the us.
In order to gain fda approval a mesothelioma clinical trial must go through three or four phases. Clinical trials are research studies that involve people. The clinical trials on this list are for mesothelioma treatment.
Clinical trial phases phases of a mesothelioma clinical trial. You need to talk to your specialist if there are any trials that you think you might be able to take part in. Researchers are looking at the causes diagnosis and treatment of mesothelioma.
More From Is Asbestos Dangerous If Not Disturbed
- Handprint Coloring Page
- Lawrence Stern Artist
- How Big Are Tumors In Mesothelioma
- Transite Roof Panels
- Free Frida Kahlo Coloring Pages
Incoming Search Terms:
- Updates In Immunotherapy For Mesothelioma Mesothelioma Applied Research Foundation Free Frida Kahlo Coloring Pages,
- Fibulin 3 As A Blood And Effusion Biomarker For Pleural Mesothelioma Nejm Free Frida Kahlo Coloring Pages,
- Mesothelioma Clinical Trials Find New Mesothelioma Treatment Free Frida Kahlo Coloring Pages,
- Phase Iii Clinical Trial For Inoperable Mesothelioma Shows Substantial Tumor Shrinkage Free Frida Kahlo Coloring Pages,
- Mesothelioma Clinical Trials Improve Prognosis With New Treatments Free Frida Kahlo Coloring Pages,
- Mesothelioma Research Institutions Usc Norris Comprehensive Cancer Center Free Frida Kahlo Coloring Pages,