Imfinzi Structure, Molecular Mechanism Of Pd 1 Pd L1 Blockade Via Anti Pd L1 Antibodies Atezolizumab And Durvalumab Scientific Reports
Imfinzi structure Indeed lately has been sought by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the name of the article I will discuss about Imfinzi Structure.
- Https Www Mdpi Com 1420 3049 24 6 1190 Pdf
- Full Text Safety And Efficacy Of Durvalumab Medi4736 In Various Solid Tumors Dddt
- Cancer Immunotherapy Wikipedia
- Durvalumab New Drug Approvals
- Https Www Ema Europa Eu Documents Variation Report Imfinzi H C 4771 Ii 0014 G Epar Assessment Report Variation En Pdf
- Molecular Mechanism Of Pd 1 Pd L1 Blockade Via Anti Pd L1 Antibodies Atezolizumab And Durvalumab Scientific Reports
Find, Read, And Discover Imfinzi Structure, Such Us:
- How Ketamine Could Help Treat Severe Depression Feature Chemistry World
- About Imfinzi Durvalumab
- Full Text Research Status And Outlook Of Pd 1 Pd L1 Inhibitors For Cancer Therap Dddt
- Https Www Ema Europa Eu Documents Variation Report Imfinzi H C 4771 Ii 0014 G Epar Assessment Report Variation En Pdf
- Hutchison China Meditech Ltd 2019 Foreign Issuer Report 6 K
- Nigel Satterley House
- Floral Coloring Pages Flowers
- Sutton Lawn Fishing
- Kazan Olympics
- Nevada Mesothelioma Lawyer
If you re looking for Nevada Mesothelioma Lawyer you've reached the right place. We have 100 images about nevada mesothelioma lawyer adding pictures, pictures, photos, backgrounds, and more. In these page, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.

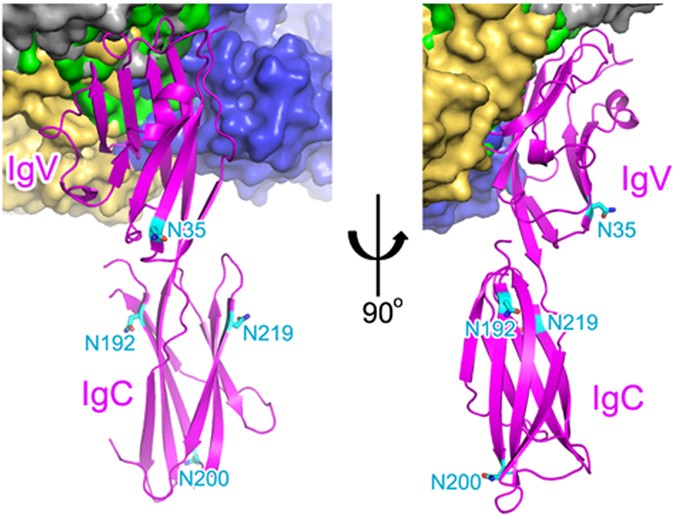
Structural Biology Of The Immune Checkpoint Receptor Pd 1 And Its Ligands Pd L1 Pd L2 Sciencedirect Nevada Mesothelioma Lawyer
Https Www Ema Europa Eu Documents Variation Report Imfinzi H C 4771 Ii 0014 G Epar Assessment Report Variation En Pdf Nevada Mesothelioma Lawyer
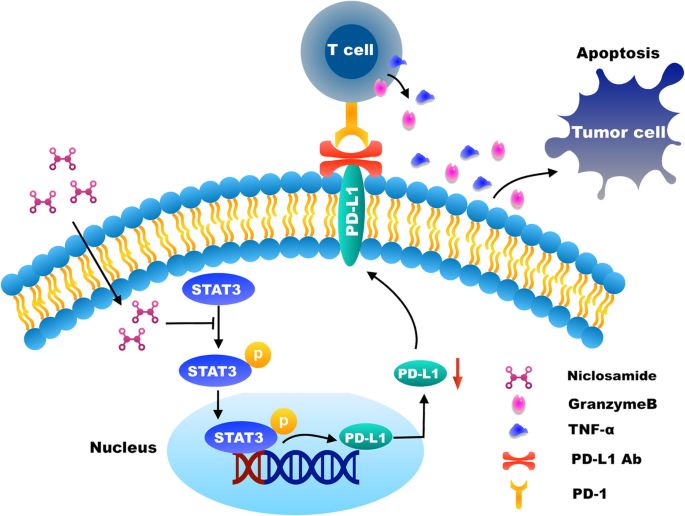
Durvalumab is the generic name for the trade name drug imfinzi.

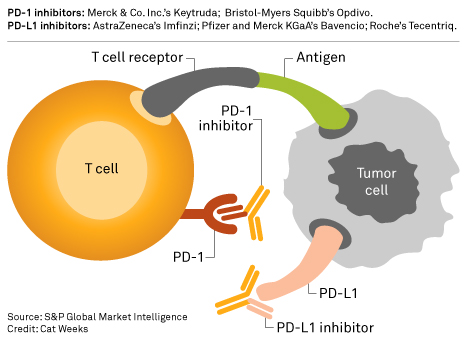
Nevada mesothelioma lawyer. This medication is classified as an anti pd l1 monoclonal antibody. It is a human immunoglobulin g1 kappa igg1k monoclonal antibody that blocks the interaction of programmed cell death ligand 1 pd l1 with the pd 1 cd279. How does imfinzi work.
Mechlorethamine is metabolized to an unstable highly reactive ethyleniminium intermediate that alkylates dna particularly the 7 nitrogen of guanine residues resulting in dna base pair mismatching dna. Imfinzi durvalumab is a monoclonal antibody a human immunoglobulin g1 kappa igg1k monoclonal antibody a type of protein that has been designed to recognise and attach to a specific structure found in certain cells in the body. Description view imfinzi description for details of the chemical structure and excipients inactive components.
In clinical studies to date involving over 1000 patients savolitinib has. Administer corticosteroids for grade 2 or greater pneumonitis. Immunotherapy drugs use the bodys own immune system to fight cancer.
Imfinzi durvalumab is a type of cancer treatment called an immunotherapy developed by astrazeneca and approved to treat certain cancers of the bladder urinary tract and lungs. A type of white blood cell called t cells that play an important role in the normal function of the immune system are. Durvalumab is known as a checkpoint inhibitor drug.
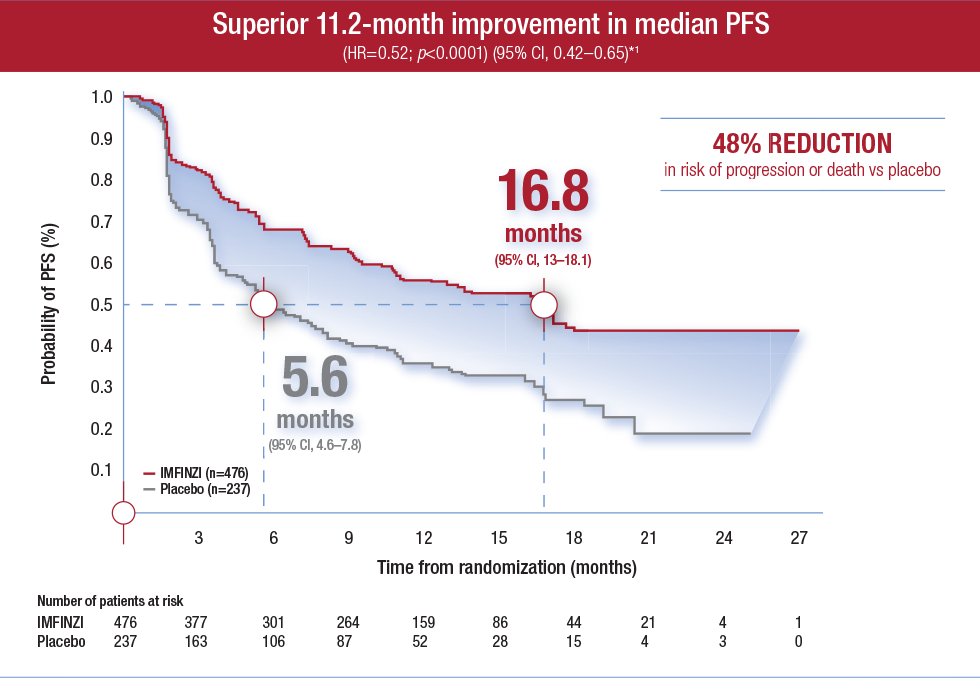
Durvalumab trade name imfinzi is an fda approved immunotherapy for cancer developed by medimmuneastrazeneca. Mechlorethamine hydrochloride is the hydrochloride salt of mechlorethamine a nitrogen mustard and an analogue of sulfur mustard with antineoplastic and immunosuppressive activities. Imfinzi reduced the risk of death by 31 percent at two years.
View imfinzi storage conditions for details to ensure optimal shelf life. Through chemical structure modification addresses human metabolite related renal toxicity the primary issue that halted development of several other selective met inhibitors. Monitor patients for signs and symptoms of pneumonitis and evaluate with radiographic imaging when suspected.
The calypso study is an independently sponsored open label phase ii study of imfinzi. In some cases health care professionals may use the trade name imfinzi when referring to the generic drug name durvalumab. The investigators said the pacific study is the first study to demonstrate a survival advantage for unresectable stage iii nsclc non small cell lung cancer which accounts for more than 80 percent of all lung cancers is often detected late and has a high mortality rate.
Fatal cases have been reported. Imfinzi may be used when your nsclc has not spread outside your chest cannot be removed by surgery and has responded or stabilized with initial treatment with chemotherapy that contains platinum given at the same time as radiation therapy.
More From Nevada Mesothelioma Lawyer
- Italian Law Firm New York
- Llp Lawyer Meaning
- Easy Pocahontas Coloring Pages
- John Mayer Nike Collab
- Meyers Cleaning Products Scents
Incoming Search Terms:
- Structural Biology Of The Immune Checkpoint Receptor Pd 1 And Its Ligands Pd L1 Pd L2 Sciencedirect Meyers Cleaning Products Scents,
- Ndc 0310 4611 Imfinzi Durvalumab Meyers Cleaning Products Scents,
- Https Www Cadth Ca Sites Default Files Pcodr Reviews2019 10131durvalumabnsclc Fnegr Noredact Abbrev Post 03may2019 Final Pdf Meyers Cleaning Products Scents,
- Hutchison China Meditech Ltd 2019 Foreign Issuer Report 6 K Meyers Cleaning Products Scents,
- Document Meyers Cleaning Products Scents,
- Https Www Mdpi Com 1420 3049 24 6 1190 Pdf Meyers Cleaning Products Scents,