Tremelimumab Mesothelioma Fda, Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcr 4yiwrullkrci1nqzl9uc1pkhr8l3u5pycep2zqhvyuag7znt Usqp Cau
Tremelimumab mesothelioma fda Indeed lately has been hunted by users around us, perhaps one of you personally. People now are accustomed to using the net in gadgets to see video and image information for inspiration, and according to the title of this article I will talk about about Tremelimumab Mesothelioma Fda.
- Astrazeneca Presents Imfinzi Durvalumab Plus Tremelimumab Combination Data At Aacr Annual Meeting Be Korea Savvy
- Https Www Tandfonline Com Doi Pdf 10 1517 14712598 2015 1116515
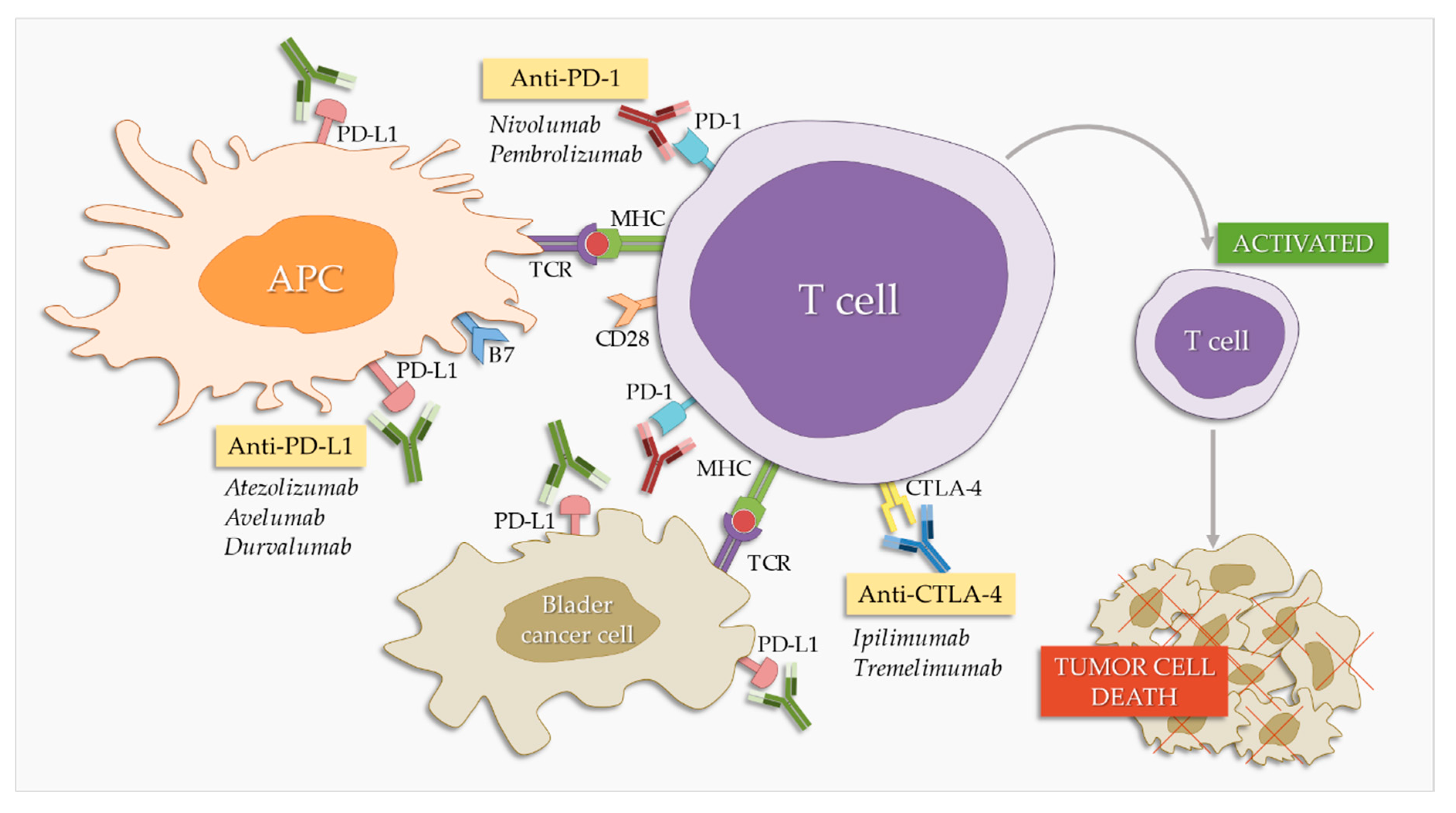
- Anti Ctla 4 Therapy For Malignant Mesothelioma Immunotherapy
- Does Immunotherapy Drug Hold The Key In The Treatment Of Mesothelioma
- Cancers Free Full Text Current Strategies And Novel Therapeutic Approaches For Metastatic Urothelial Carcinoma Html
- Mesothelioma Treatment Are We On Target A Review Sciencedirect
Find, Read, And Discover Tremelimumab Mesothelioma Fda, Such Us:
- Packaging String Definition Tremelimumab Package Insert
- Tremelimumab Receives Orphan Drug Designation From Fda For Malignant Mesothelioma Treatment Baron Budd Reports
- Similar Epitope And Distinct Binding Mode Of Ipilimumab And Download Scientific Diagram
- Full Text Safety And Efficacy Of Durvalumab Medi4736 In Various Solid Tumors Dddt
- Medimmune News Articles Etc European Pharmaceutical Review
- Is 2 Asbestos Dangerous
- Sokolov Mesothelioma
- Myers Park Neighborhood
- Mesothelioma Causes Mayo Clinic
- Simmons Beautyrest Platinum Sandy Springs In Luxury Firm
If you are searching for Simmons Beautyrest Platinum Sandy Springs In Luxury Firm you've come to the perfect place. We have 104 graphics about simmons beautyrest platinum sandy springs in luxury firm adding pictures, pictures, photos, wallpapers, and much more. In such page, we also provide variety of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.

Astrazeneca Misses Endpoint In Big Checkpoint Inhibitor Combo Trial Simmons Beautyrest Platinum Sandy Springs In Luxury Firm

Pdf Tremelimumab Research And Clinical Development Simmons Beautyrest Platinum Sandy Springs In Luxury Firm
The uk based pharmaceutical company astrazeneca announced on wednesday that the us.

Simmons beautyrest platinum sandy springs in luxury firm. The study conducted from oct 30 2015 to oct 12 2016 consisted of 40 mesothelioma patients. In the companys press release robert iannone head of immuno oncology at astrazenecas global medicines development division said that there is a crucial need to develop. Orphan drug designation is not the same as fda approval but it does help pharmaceutical companies.
In april mesothelioma help announced the fda granted orphan drug designation for tremelimumab for the treatment of malignant mesotheliomathe drug was found to increase survival in mesothelioma patients. The goal of this clinical trial is to test the safety and the effectiveness of both durvalumab and tremelimumab. Researchers are studying the combination of these two immunotherapy drugs and are comparing their findings to the usual approaches that are used for treating malignant pleural mesothelioma.
As discussed in a recent article from fda news alert for patients a mesothelioma diagnosis is one of the scariest things they can hear. Astrazenecas tremelimumab receives fda approval to treat mesothelioma. Tremelimumab which has no brand name yet has not been approved by the us.
When combined with durvalumab brand name imfinzi an fda approved immunotherapy drug for cancer patients had a better response. New therapeutic strategies for malignant mesothelioma are urgently needed. Now in a new clinical trial researchers are assessing whether the combination of tremelimumab and durvalumab for lung cancer treatment will fight the cancer better than either drug alone.
Mesothelioma is a rare and aggressive cancer that affects the lining of the lungs and abdomen and limited treatments are available for this cancer. Food drug administration fda has granted tremelimumab orphan drug status for the treatment of malignant mesothelioma. Each participant received at least one dose each of tremelimumab and durvalumab.
Food and drug administration fda to treat any cancer or disease. Tremelimumab is one of several drugs that are in development as a treatment for malignant mesothelioma. It is a rare illness and there is no cure.
In the determine study we investigated the effects of the cytotoxic t lymphocyte associated antigen 4 ctla 4 monoclonal antibody tremelimumab in patients with previously treated advanced malignant mesothelioma. Astrazeneca today announced that the us food and drug administration has granted orphan drug designation for the anti ctla 4 monoclonal antibody tremelimumab for the treatment of malignant mesothelioma. Malignant mesothelioma impacts about 3000 americans each year.
Earlier this year the fda granted orphan drug status to tremelimumab for the treatment of mesothelioma. Mesothelioma is a rare aggressive cancer that most often affects the lining of the lungs and abdomen.

Astrazeneca Tremelimumab Trial Fails To Meet Endpoints Simmons Beautyrest Platinum Sandy Springs In Luxury Firm
More From Simmons Beautyrest Platinum Sandy Springs In Luxury Firm
- Riverbank Park New York
- Halloween Costume Ideas Pictures
- Mesothelioma Risk Groups
- Mesothelioma Surgery Nz
- Polio Vaccine And Mesothelioma
Incoming Search Terms:
- Enhancing Dendritic Cell Therapy In Solid Tumors With Immunomodulating Conventional Treatment Molecular Therapy Oncolytics Polio Vaccine And Mesothelioma,
- Tremelimumab A Review Of Development To Date In Solid Tumors Immunotherapy Polio Vaccine And Mesothelioma,
- Immunotherapy For Mesothelioma A Critical Review Of Current Clinical Trials And Future Perspectives Gray Translational Lung Cancer Research Polio Vaccine And Mesothelioma,
- Clinical Trial Of Imfinzi Durvalumab In Conjunction With Chemotherapy Yields Significantly Longer Survival Time For Patients Diagnosed With Malignant Pleural Mesothelioma Polio Vaccine And Mesothelioma,
- Astrazeneca S Tremelimumab Receives Fda Approval To Treat Mesothelioma Polio Vaccine And Mesothelioma,
- Mesothelioma Causes Symptoms Diagnosis Treatment Prevention Polio Vaccine And Mesothelioma,