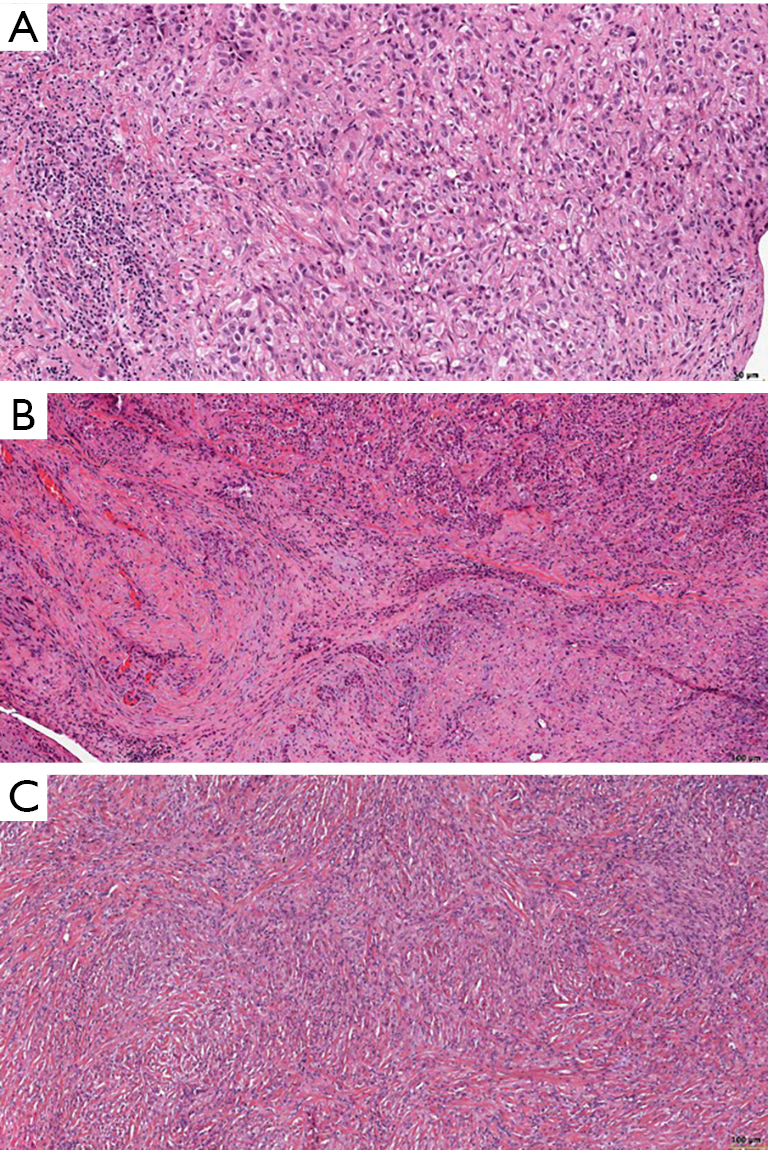
Tazemetostat Mesothelioma, What Can We Offer A Patient With A Malignant Mesothelioma Iaslc
Tazemetostat mesothelioma Indeed lately has been hunted by users around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to see image and video data for inspiration, and according to the title of the article I will talk about about Tazemetostat Mesothelioma.
- Bap1 And Yy1 Regulate Expression Of Death Receptors In Malignant Pleural Mesothelioma Biorxiv
- Tazemetostat Shows Promise For Pleural Mesothelioma
- Sec Filing Epizyme Inc
- You Should Not Miss Epizyme In 2018 Nasdaq Epzm Seeking Alpha
- Potential Mesothelioma Drug Moves One Step Closer To The Market
- Pipeline Epizyme
Find, Read, And Discover Tazemetostat Mesothelioma, Such Us:
- Https Www Fda Gov Media 133575 Download
- Novel Systemic Therapy Against Malignant Pleural Mesothelioma Mancuso Translational Lung Cancer Research
- Epizyme Halts Phase Ii Trial Of Tazemetostat In Dlbcl Continues Talks To Lift Clinical Hold
- Https Www Inoncology Com Sites Default Files Fpp Download Elcc 2018 Fennell Presentation 0 Pdf
- Sid 178103590 Pubchem
- Community Legal Services Find A Solicitor
- Coloring Pages For Girl Tweens
- Christmas Tree Coloring
- Silly Monster Coloring Pages
- Simmons Beautyrest Platinum Phillipsburg Iii
If you re searching for Simmons Beautyrest Platinum Phillipsburg Iii you've reached the ideal location. We ve got 100 graphics about simmons beautyrest platinum phillipsburg iii adding images, photos, photographs, wallpapers, and more. In such page, we also have number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.

Jci Insight Inhibition Of Ezh2 Methyltransferase Decreases Immunoediting Of Mesothelioma Cells By Autologous Macrophages Through A Pd 1 Dependent Mechanism Simmons Beautyrest Platinum Phillipsburg Iii
International Symposium On Mesothelioma To Focus On Immunotherapy Simmons Beautyrest Platinum Phillipsburg Iii
The manufacturer of tazemetostat has now formally begun its planned phase 2 clinical trial of this novel targeted mesothelioma therapeutic.
Simmons beautyrest platinum phillipsburg iii. In part 1 12 subjects with relapsed or refractory malignant mesothelioma regardless of bap1 status will be treated and undergo pharmacokinetics pk blood sample collection after a single tazemetostat 800 mg. Standard of care treatment for mesothelioma typically involves a combination of chemotherapy surgery and radiation but the effectiveness has been limited. In part 1 12 patients with relapsed or refractory malignant mesothelioma regardless of bap1 status will be treated with a single dose of tazemetostat to assess its pharmacokinetics.
There is no cure for mesothelioma and patients typically survive only nine to 18 months after being diagnosed. According to lead investigator marjorie glass zauderer md ms facp memorial sloan kettering cancer center new york and colleagues preclinical data shave shown that inactivation of bap1. Tazemetostat is reported to be capable of inhibiting ezh2 activity.
Each patient in this trial received tazemetostat twice daily. Food and drug administration for the treatment of mesothelioma but its effectiveness is limited. Screening of subjects to determine eligibility for the study will be performed within 21 days of the first planned dose of tazemetostat.
A possible new drug for mesothelioma has received fda approval for the treatment of advanced epithelioid sarcoma. Chemotherapy with cisplatin and pemetrexed is approved by the us. It is a brand new kind of drug that blocks a protein called ezh2.
Less than one fourth of. Tazemetostat tazverik is an oral medication made by a company called epizyme. In a phase 2 study tazemetostat was well tolerated and demonstrated anti tumor activity in patients with bap1 deficient relapsed or refractory malignant mesothelioma j clin onco.
How tazemetostat works for mesothelioma treatment. Phase 2 trial data being presented at the virtual 2020 asco annual meeting demonstrate the antitumor activity of tazemetostat in patients with bap1 deficient relapsed or refractory malignant mesothelioma. Abstr 9058preclinical data showed that bap1 inactivation sensitizes mesothelial cells to inhibition of ezh2 wrote marjorie glass zauderer md memorial sloan kettering cancer center new.
Epizyme inc based in cambridge massachusetts announced in mid august 2016 that the first of an expected 67 patients began receiving tazemetostat. While the gene therapy didnt lead to a complete disease response it did help stop mesotheliomas growth in many cases. In part 2 patients with bap1 mutated relapsed or refractory malignant mesothelioma will receive oral tazemetostat twice daily until disease progression.

Bap1 And Yy1 Regulate Expression Of Death Receptors In Malignant Pleural Mesothelioma Biorxiv Simmons Beautyrest Platinum Phillipsburg Iii
More From Simmons Beautyrest Platinum Phillipsburg Iii
- What Does Mesothelioma Belly Pain Feel Like
- Colouring Pictures For Girls Cat
- Ecourts Attorney Search
- Most Prestigious Law Firms Nyc
- Lung Cancer Genetic
Incoming Search Terms:
- Tazemetostat Epz 6438 Ezh2 Inhibitor Medchemexpress Lung Cancer Genetic,
- Fda Orders Enrollment Hold On Tazemetostat Clinical Trials Lung Cancer Genetic,
- Epizyme S Tazverik Receives Accelerated Approval From Fda Lung Cancer Genetic,
- Recruiting Mesothelioma Clinical Trials Get Enrolled Lung Cancer Genetic,
- Mesothelioma Scientific Clues For Prevention Diagnosis And Therapy Carbone 2019 Ca A Cancer Journal For Clinicians Wiley Online Library Lung Cancer Genetic,
- Mesothelioma Scientific Clues For Prevention Diagnosis And Therapy Carbone 2019 Ca A Cancer Journal For Clinicians Wiley Online Library Lung Cancer Genetic,