Pleural Mesothelioma Keytruda, Mesothelioma And Keytruda Success Rate Side Effects More
Pleural mesothelioma keytruda Indeed recently has been hunted by consumers around us, maybe one of you. Individuals now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the name of the article I will talk about about Pleural Mesothelioma Keytruda.
- Opdivo Archives Mesothelioma Applied Research Foundation
- Https Www Bc Legal Co Uk Images Keytruda 20treatment 20costs 20in 20mesothelioma 20claims 20bcdn 20version Pdf
- Another Win For Mesothelioma Immunotherapy Drug Keytruda
- Keytruda In Advanced Pleural Mesothelioma Data Presented
- Pdf Retrospective Evaluation Of The Use Of Pembrolizumab In Malignant Mesothelioma In A Real World Australian Population
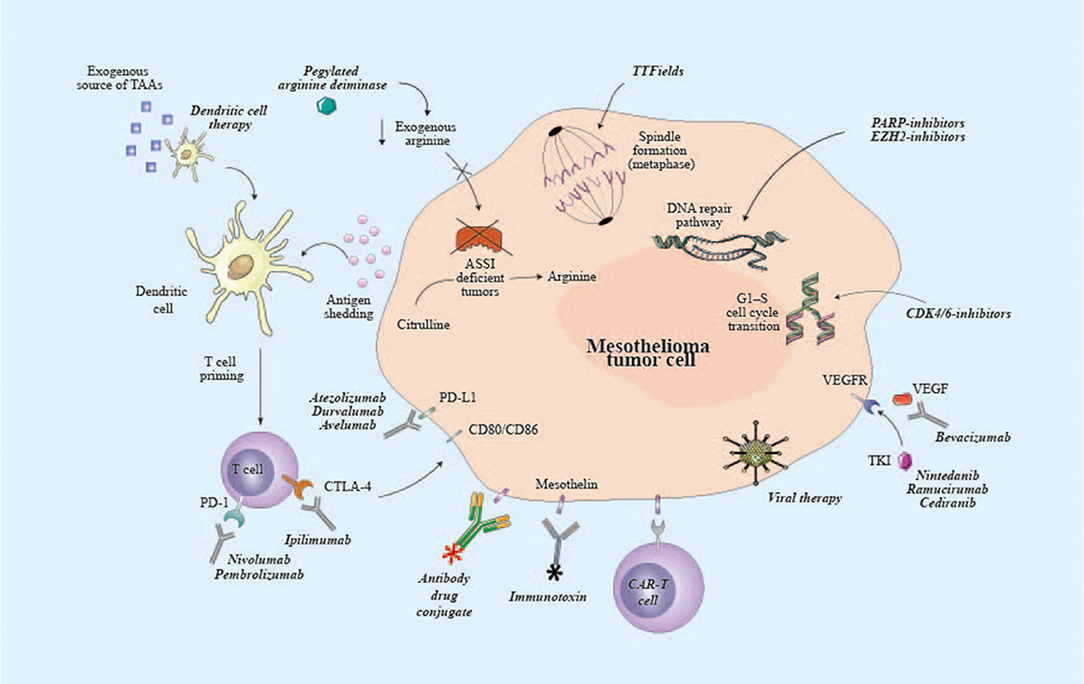
- Frontiers Malignant Pleural Mesothelioma State Of The Art On Current Therapies And Promises For The Future Oncology
Find, Read, And Discover Pleural Mesothelioma Keytruda, Such Us:
- Keytruda In Advanced Pleural Mesothelioma Data Presented
- Opdivo Archives Mesothelioma Applied Research Foundation
- Ipilimumab And Nivolumab In The Treatment Of Recurrent Malignant Pleural Mesothelioma Initiate Results Of A Prospective Single Arm Phase 2 Trial The Lancet Respiratory Medicine
- Keytruda Survival Benefits Similar To Chemo For Relapsed Mesothelioma Phase 3 Trial Finds
- Malignant Pleural Mesothelioma Disease Malacards Research Articles Drugs Genes Clinical Trials
- Local Attorneys
- Mesothelioma Treatment Cisplatin And Pemetrexed
- Coding For Pleural Mesothelioma
- Cool Coloring Pages Printable
- Posable Skeleton
If you are looking for Posable Skeleton you've reached the right place. We ve got 100 graphics about posable skeleton including images, pictures, photos, backgrounds, and much more. In these page, we additionally have variety of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.

Clinical Trials Eligibility Of Patients With Malignant Pleural Mesothelioma Use Of Novel Therapies And Outcomes Sciencedirect Posable Skeleton
Keytruda did not improve progression free survival for mesothelioma patients who progressed after first line chemotherapy.

Posable skeleton. There are now more than ninety on going clinical trials using keytruda to treat various types of cancers. None of the patients in the study discontinued treatment as a result of side effects. Keytruda is paving the way to a new strategy for better treatment of mesothelioma.
The keynote 028 clinical trial conducted in 13 different sites across six different countries was the first to show success using keytruda to treat malignant pleural mesothelioma patients who were previously treated with chemotherapy or were considered ineligible for treatment. Results from a phase iii clinical trial comparing keytruda pembrolizumab to standard chemotherapy shows the immunotherapy drug still has a long way to go as a viable treatment option for malignant pleural mesothelioma. Keytruda pembrolizumab the companys anti pd 1 therapy in 25 patients with advanced pleural mesothelioma a difficult to treat cancer of the lining of the lungs abdomen and other organs.
A clinical benefit of 40 percent observed during the study including both partial responses and a stable. The early findings presented showed an overall response rate confirmed and unconfirmed of 28 percent with keytruda in patients with tumors that. Immunotherapy does not offer a cure for mesothelioma but it combines well with other therapies to manage pleural mesothelioma better as a chronic disease.
The novottf 100l system device which has been rebranded optune lua received fda approval in may 2019 for specific cases of pleural mesothelioma. Keytruda is an immunotherapy drug that is designed to boost the immune system causing an anti cancer effect that can help the body kill cancer cells ultimately working to treat cancers like malignant mesotheliomathe drug developed by merck has shown promise in improving quality of life and overall survival for mesothelioma patients in clinical trials and has recently become a more. Researchers reported that 14 of 25 participants 56 experienced.
If you have pleural mesothelioma and wish to learn if youre eligible for the fdas keytruda treatment guidelines email our patient advocates. Approval for keytruda expands hope for mesothelioma patients. Promise meso nct02991482 is a phase 3 trial comparing the efficacy of keytruda to standard chemotherapy in people with advanced malignant pleural mesothelioma a lethal form of cancer in the pleura the protective lining around the lungs who failed to respond to a previous course of platinum based chemotherapy.
Pleural mesothelioma patients who took part in a keytruda clinical trial at the abramson cancer center at penn medicine reported only dry mouth fatigue nausea and decreased appetite.
More From Posable Skeleton
- Cck Law Firm
- Toy Story 4 Colouring Pages
- Printable Coloring Images
- Lung Cancer Ct Scan Images
- Color By Number Mandala Printable
Incoming Search Terms:
- Petition Federal Health Minister Sussan Ley Keytruda On Pbs Pharmaceutical Benefits Scheme For Mesothelioma Life Saving Drug Change Org Color By Number Mandala Printable,
- Keytruda For Mesothelioma Flops In Phase Iii Clinical Trial Color By Number Mandala Printable,
- Malignant Pleural Mesothelioma Disease Malacards Research Articles Drugs Genes Clinical Trials Color By Number Mandala Printable,
- Pdf Retrospective Evaluation Of The Use Of Pembrolizumab In Malignant Mesothelioma In A Real World Australian Population Color By Number Mandala Printable,
- Pdf Brief Report Pembrolizumab As Palliative Immunotherapy In Malignant Pleural Mesothelioma Color By Number Mandala Printable,
- Two Powerhouse Immunotherapies Combined In Mesothelioma Trial Color By Number Mandala Printable,