Pemetrexed Carboplatin Bevacizumab Mesothelioma, A Review Of Bevacizumab In The Treatment Of Malignant Pleural Mesothelioma Semantic Scholar
Pemetrexed carboplatin bevacizumab mesothelioma Indeed lately has been hunted by consumers around us, perhaps one of you. Individuals are now accustomed to using the internet in gadgets to view image and video data for inspiration, and according to the title of the article I will talk about about Pemetrexed Carboplatin Bevacizumab Mesothelioma.
- A Multicenter Phase Ii Study Of Cisplatin Pemetrexed And Bevacizumab In Patients With Advanced Malignant Mesothelioma Semantic Scholar
- Malignant Pleural Mesothelioma Updates
- Full Text Spotlight On Bevacizumab And Its Potential In The Treatment Of Maligna Ott
- A Multicenter Phase Ii Study Of Cisplatin Pemetrexed And Bevacizumab In Patients With Advanced Malignant Mesothelioma Semantic Scholar
- Synergistic Antitumor Activity Of Recombinant Human Apo2l Tumor Necrosis Factor Related Apoptosis Inducing Ligand Trail In Combination With Carboplatin And Pemetrexed In Malignant Pleural Mesothelioma Journal Of Thoracic Oncology
- Bevacizumab For Newly Diagnosed Pleural Mesothelioma In The Mesothelioma Avastin Cisplatin Pemetrexed Study Maps A Randomised Controlled Open Label Phase 3 Trial The Lancet
Find, Read, And Discover Pemetrexed Carboplatin Bevacizumab Mesothelioma, Such Us:
- Malignant Pleural Mesothelioma Updates
- First Line Nivolumab Ipilimumab Combination Improves Os In Mesothelioma Subset
- Pdf Pemetrexed Maintenance Therapy Following Bevacizumab Containing First Line Chemotherapy In Advanced Malignant Pleural Mesothelioma A Case Report And Literatures Review
- A Review Of Bevacizumab In The Treatment Of Malignant Pleural Mesothelioma Semantic Scholar
- Actualizacion Mesotelioma Ppt Download
- Sarcomatoid Mesothelioma Pathology
- Halloween Word Search Pdf
- Printable Mandalas For Adults
- Mesothelioma First Discovered
- Mesothelioma Claims In Canada
If you re looking for Mesothelioma Claims In Canada you've reached the perfect place. We ve got 100 images about mesothelioma claims in canada adding images, photos, photographs, backgrounds, and much more. In such web page, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

Pdf Multicenter Phase Ii Study On Cisplatin Pemetrexed And Bevacizumab Followed By Maintenance With Pemetrexed And Bevacizumab For Patients With Advanced Or Recurrent Nonsquamous Non Small Cell Lung Cancer Map Study Mesothelioma Claims In Canada
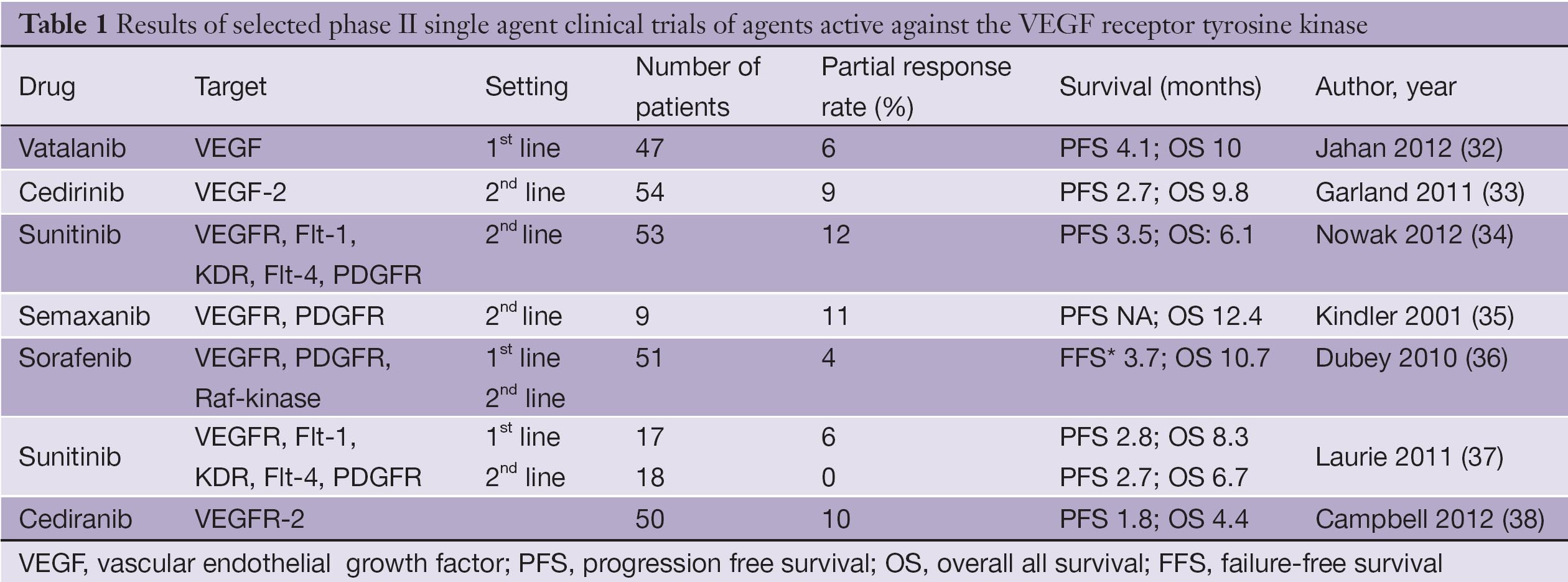
A phase ii study clin lung cancer.

Mesothelioma claims in canada. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study maps. However in our study patient subgroups that have previously shown poor. The aim of this open label phase ii study nct00407459 was to assess the activity of the vascular endothelial growth factor vegf inhibitor bevacizumab combined with pemetrexed and carboplatin in patients with previously untreated unresectable malignant pleural mesothelioma mpm.
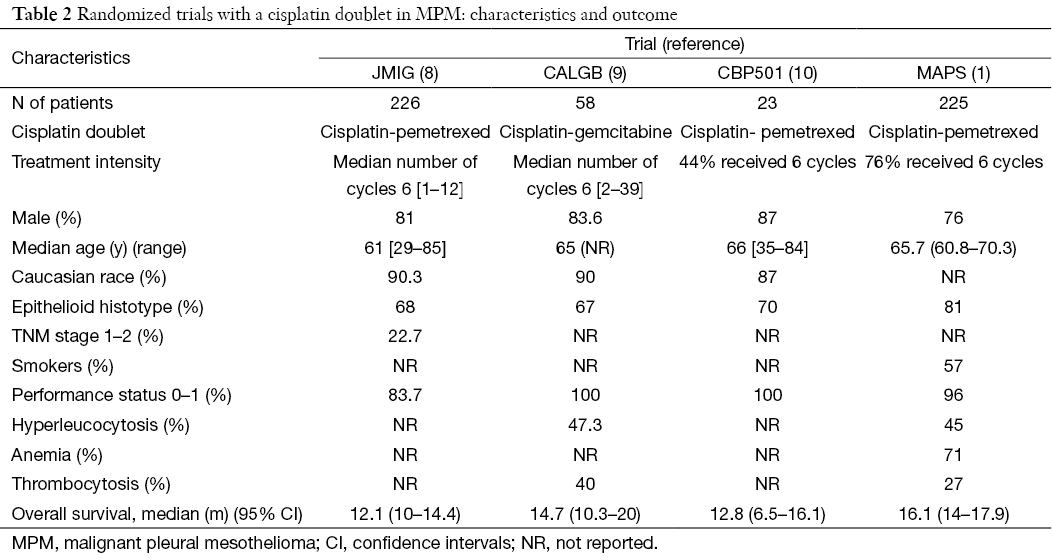
Based on the results of the phase iii ifct 0701 mesothelioma avastin cisplatin pemetrexed study cisplatin pemetrexed bevacizumab is now the accepted standard in france. Eligible patients received pemetrexed 500 mg m 2 carboplatin area under the plasma. Patients were enrolled in a cohort of 3.
Findings from our study of bevacizumab plus pemetrexed and cisplatin compared with pemetrexed and cisplatin alone showed a significantly improved median os and progression free survival with addition of bevacizumab. Eligible patients with unresectable pleural mesothelioma received frontline treatment consisting of carboplatin auc 5 bevacizumab 15 mgkg and pemetrexed 500 mgm2 every 21 days tier 1. This was a planned phase iii dose escalation study.
Chemotherapy naive patients with measurable disease and adequate organ function who were not eligible for curative surgery received pemetrexed 500 mgm2 and carboplatin area. A randomised controlled open label phase 3 trial lancet. Mesothelioma traditionally has a poor prognosis.
Phase ii study of bevacizumab pemetrexed and carboplatin as first line therapy in malignant pleural mesothelioma the safety and scientific validity of this study is the responsibility of the study sponsor and investigators. This multicenter phase ii clinical study was conducted to evaluate the activity of the combination of pemetrexed and carboplatin in patients with malignant pleural mesothelioma mpm. The role of bevacizumab in mpm was further evaluated in the french mesothelioma avastin cisplatin pemetrexed study maps31 the trial was started as a phase ii study that evaluated the disease control rate at 6 months primary endpoint and safety secondary endpoint with the addition of bevacizumab to cisplatin and pemetrexed.
14 bevacizumab addition to pemetrexedcisplatin provides a significantly. Epub 2015 dec 21. Carboplatin plus pemetrexed as first line treatment of patients with malignant pleural mesothelioma.
More From Mesothelioma Claims In Canada
- What Kind Of Test Can Be Done To Detect Mesothelioma
- Medical Lawyers Salary
- New York Patent Law Firms
- Colorfy Online
- Color By Number Sheets
Incoming Search Terms:
- Health Related Quality Of Life In Patients With Advanced Nonsquamous Non Small Cell Lung Cancer Receiving Bevacizumab Or Bevacizumab Plus Pemetrexed Maintenance Therapy In Avaperl Mo22089 Journal Of Thoracic Oncology Color By Number Sheets,
- Malignant Mesothelioma Thoracic Key Color By Number Sheets,
- Pdf Bevacizumab For Newly Diagnosed Pleural Mesothelioma In The Mesothelioma Avastin Cisplatin Pemetrexed Study Maps A Randomised Controlled Open Label Phase 3 Trial Semantic Scholar Color By Number Sheets,
- Medical Treatment Of Malignant Pleural Mesothelioma Relapses Petrini Journal Of Thoracic Disease Color By Number Sheets,
- Treatment Of Malignant Pleural Mesothelioma American Society Of Clinical Oncology Clinical Practice Guideline Kindler Hl Et Al Ppt Download Color By Number Sheets,
- Mesothelioma A Review Abstract Europe Pmc Color By Number Sheets,