Pembrolizumab Mesothelioma Fda, Keynote 042 No Hard And Fast Rules For First Line Pembrolizumab Regarding Pd L1 Positive Disease Iaslc
Pembrolizumab mesothelioma fda Indeed recently is being hunted by users around us, perhaps one of you personally. Individuals now are accustomed to using the net in gadgets to see image and video data for inspiration, and according to the title of the article I will talk about about Pembrolizumab Mesothelioma Fda.
- Fda Approves Keytruda For Certain Mesothelioma Patients
- Fda Approval Of Keytruda Will Help Some Mesothelioma Patients
- Keytruda Case Study Offers Home For Advanced Mesothelioma
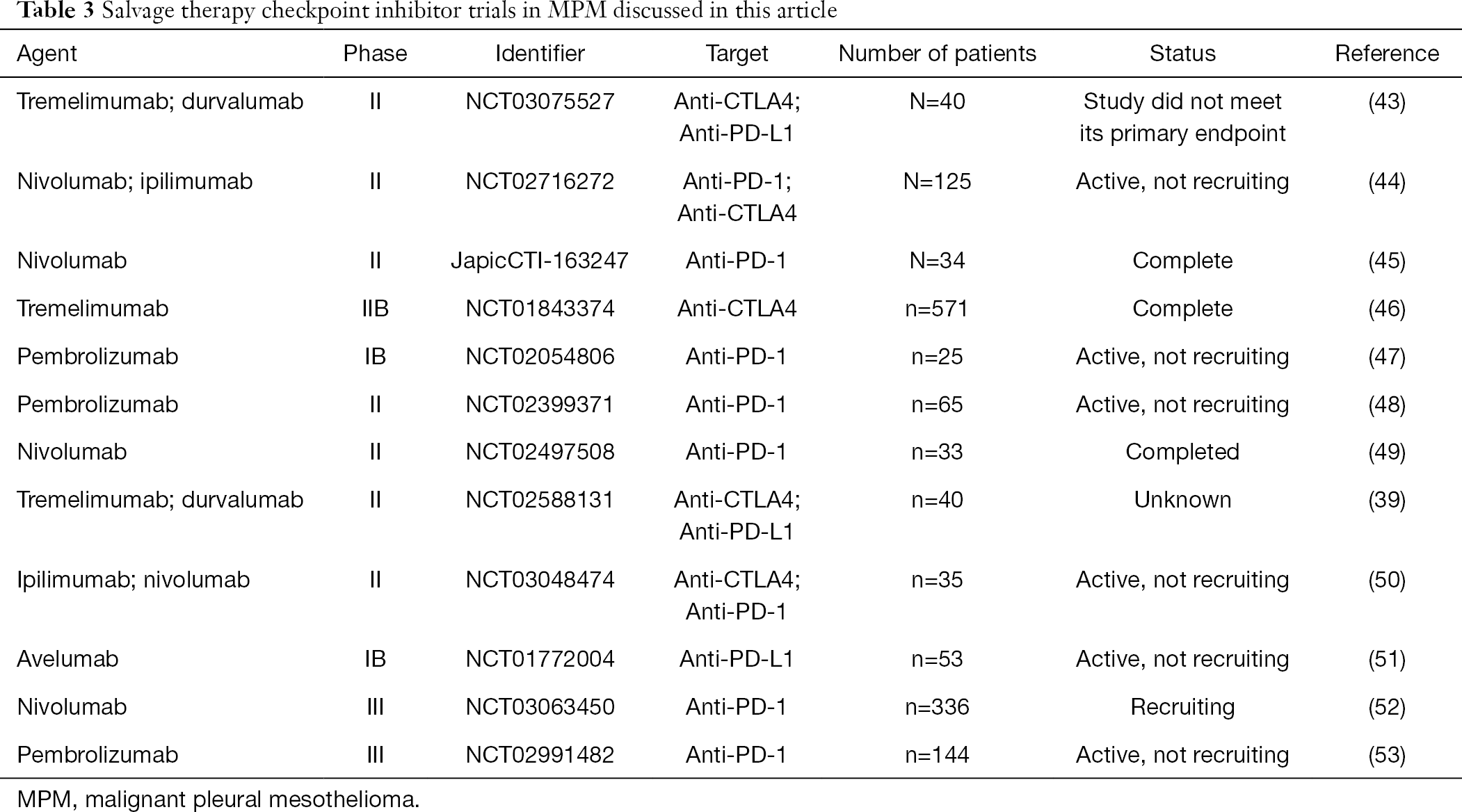
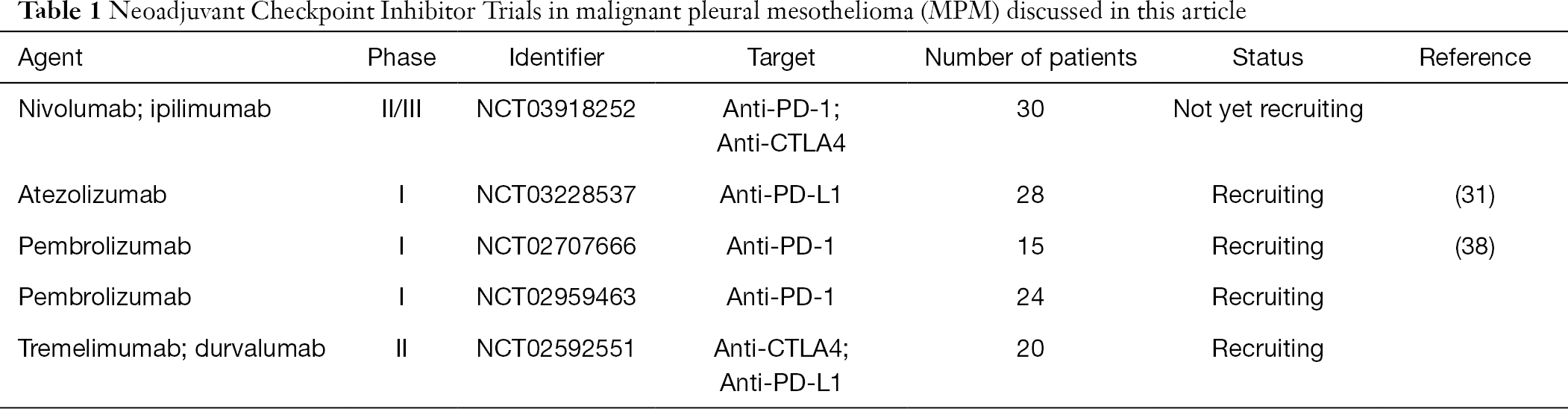
- Immunotherapy For Mesothelioma A Critical Review Of Current Clinical Trials And Future Perspectives Gray Translational Lung Cancer Research
- Keynote 042 No Hard And Fast Rules For First Line Pembrolizumab Regarding Pd L1 Positive Disease Iaslc
- Pdf Significance And Implications Of Fda Approval Of Pembrolizumab For Biomarker Defined Disease
Find, Read, And Discover Pembrolizumab Mesothelioma Fda, Such Us:
- Pdf Retrospective Evaluation Of The Use Of Pembrolizumab In Malignant Mesothelioma In A Real World Australian Population
- Https Www Bc Legal Co Uk Images Keytruda 20treatment 20costs 20in 20mesothelioma 20claims 20bcdn 20version Pdf
- Mesothelioma Trial Suggests Immunotherapy As An Alternative To Chemotherapy
- Cautious Optimism The Current Role Of Immunotherapy In Gastrointestinal Cancers
- Keytruda Approved For Mesothelioma In Specific Cases
- Roger Plowman Mesothelioma
- Little Drummer Boy Coloring Page
- New York Nursing Home Abuse Lawyer
- Knoxville Mesothelioma Attoreny
- Mesothelioma Dlco Change Usmle
If you re looking for Mesothelioma Dlco Change Usmle you've come to the right place. We ve got 100 graphics about mesothelioma dlco change usmle including images, photos, pictures, backgrounds, and much more. In such web page, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
Keytruda is the brand name of the drug pembrolizumab which has been featured in a number of mesothelioma clinical trials.
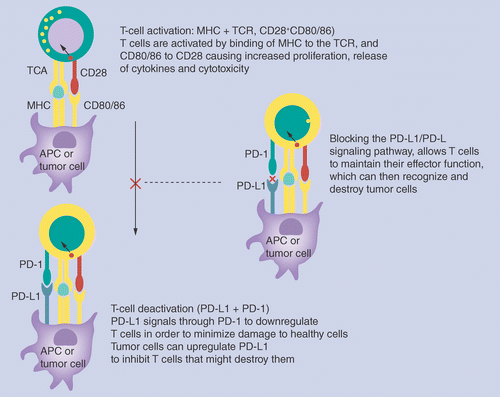
Mesothelioma dlco change usmle. On june 29 2020 the food and drug administration approved pembrolizumab keytruda merck co for the first line treatment of patients with unresectable or metastatic microsatellite. On april 28 2020 the food and drug administration granted accelerated approval to a new dosing regimen of 400 mg every six weeks for pembrolizumab keytruda merck across all currently approved. 1 2 for people affected by asbestos exposure keytruda is exciting because early studies suggest it may be a useful chemotherapy drug for mesothelioma.
Keytruda pembrolizumab is approved to treat cancers with a specific biomarker the first drug to be approved by the us. The fdas approval occurred tuesday june 16 2020. This latest fda approval is for the treatment of.
Food and drug administration approved the use of pembrolizumab for certain metastatic tumors this week. Pembrolizumab often known by the brand name keytruda is a well known immunotherapy drug already being used with mixed success for several cancers. Following confirmation of positive results the us.
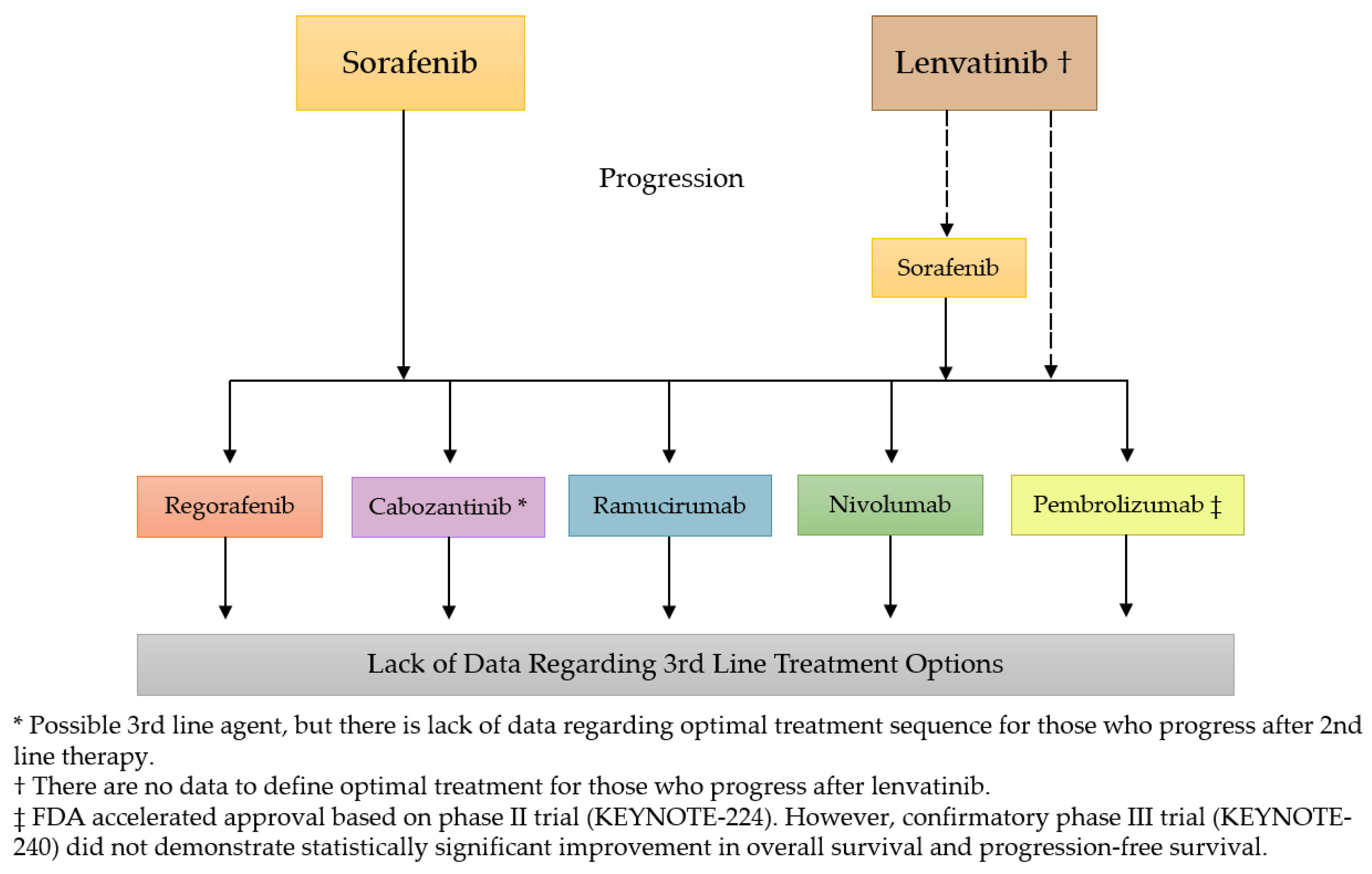
Keytruda is an immunotherapy drug categorized as an immune checkpoint inhibitor a type of monoclonal antibody. Patients with pleural mesothelioma cancer may have another treatment option after the us. Keytruda did not improve progression free survival for mesothelioma patients who progressed after first line chemotherapy.
The drug works by blocking the pd l1 protein which is a protein that can be found on normal and cancer cells. Food and drug administration fda expanded its approval of pembrolizumab for first line treatment of metastatic non small cell lung cancer last week moving it closer to becoming a viable treatment option for patients with pleural mesothelioma. Overview how keytruda treats mesothelioma.
This page lists cancer drugs approved by the food and drug administration fda for malignant mesothelioma. Food and drug administration fda based on cancer genetics. It helps the bodys immune system detect and destroy cancer cells.
The united states food and drug administration fda approved the brand name immunotherapy drug for specific cases of pleural mesothelioma among other solid tumor cancers. Results from a phase iii clinical trial comparing keytruda pembrolizumab to standard chemotherapy shows the immunotherapy drug still has a long way to go as a viable treatment option for malignant pleural mesothelioma. The drug names link to ncis cancer drug information summaries.
Pembrolizumab is marketed by merck co. The list includes generic names and brand names.
More From Mesothelioma Dlco Change Usmle
- Secret Garden Coloring Pages Printable
- Preschool Valentines Day Coloring Pages
- Mesothelioma Cell Line Types
- Rodlesberger Study Mesothelioma
- Coloring Page For 2 Year Old
Incoming Search Terms:
- Immunotherapy For Mesothelioma A Critical Review Of Current Clinical Trials And Future Perspectives Gray Translational Lung Cancer Research Coloring Page For 2 Year Old,
- Https Www Bc Legal Co Uk Images Keytruda 20treatment 20costs 20in 20mesothelioma 20claims 20bcdn 20version Pdf Coloring Page For 2 Year Old,
- Is Keytruda Fda Approved For Mesothelioma Coloring Page For 2 Year Old,
- Latest Keytruda Approval Promising For Mesothelioma Patients Coloring Page For 2 Year Old,
- Mesothelioma Drugs Flagged On Fda S Quarterly Adverse Events Reports Coloring Page For 2 Year Old,
- Philadelphia Mesothelioma Lawyers Important Update On Keytruda Coloring Page For 2 Year Old,