Nvcr Fda Approval Mesothelioma, Major Biopharma Catalysts On September S Fda Calendar
Nvcr fda approval mesothelioma Indeed lately has been sought by consumers around us, maybe one of you. People now are accustomed to using the net in gadgets to view image and video information for inspiration, and according to the name of the post I will talk about about Nvcr Fda Approval Mesothelioma.
- Https Www Novocure Com Wp Content Uploads 2019 01 201901 Nvcr Corporate Presentation Vf 1 Pdf
- Http 3sj0u94bgxp33grbz1fkt62h Wpengine Netdna Ssl Com Wp Content Uploads 2019 10 201908 Nvcr Corporate Presentation V2 0 Pdf
- Https 3sj0u94bgxp33grbz1fkt62h Wpengine Netdna Ssl Com Wp Content Uploads 2018 11 Nvcr 201811 Corporate Presentation Vf Pdf
- Results From Stellar Trial Of Tumor Treating Fields With Chemotherapy In Malignant Pleural Mesothelioma Published In The Lancet Oncology Business Wire
- Novocure Touts Top Line Data From Ph2 Mesothelioma Trial Drug Delivery Business
- Novocure Ltd Nasdaq Nvcr Releases Big Announcement
Find, Read, And Discover Nvcr Fda Approval Mesothelioma, Such Us:
- Nvcr Nerv Stock Quote
- 2
- Novocure Receives Ce Mark For Novottf 100l System
- Results From Stellar Trial Of Tumor Treating Fields With Chemotherapy In Malignant Pleural Mesothelioma Published In The Lancet Oncology Business Wire
- Why Novocure S Regulatory News Is A Huge Win For Investors The Motley Fool
- Hyatt And Hyatt Law Firm Miami
- Motorcycle Accident Attorney Sacramento
- Toddler Black History Coloring Pages
- Elmo Coloring Pages To Print
- Quartz Wool Mesothelioma
If you are searching for Quartz Wool Mesothelioma you've arrived at the ideal location. We have 100 images about quartz wool mesothelioma adding images, pictures, photos, wallpapers, and much more. In these web page, we also have number of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
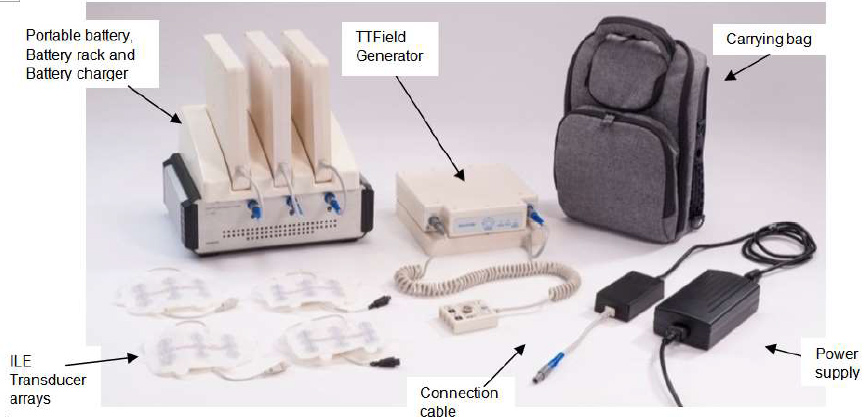
Novottf 100l is a non invasive antimitotic cancer treatment designed to deliver electric fields at special frequencies to disrupt solid tumor cancer cell division.

Quartz wool mesothelioma. Novocure nsdqnvcr announced that it has received fda approval for its novottf 100l system with chemotherapy for treating a type of mesothelioma. Helier jerseybusiness wire novocure nasdaq. Food and drug administration fda has approved the novottf 100l system in combination with pemetrexed plus platinum based chemotherapy for the first line treatment of unresectable locally advanced or metastatic malignant pleural mesothelioma mpm.
Last year the company received fda approval via the humanitarian device exemption hde pathway for the treatment of adults with malignant pleural mesothelioma the companys first fda approved. Fda approves drug combination for treating mesothelioma first approval in 16 years for mesothelioma a type of cancer caused by inhaling asbestos fibers. Food and drug administration fda recently announced approval of an immunotherapy treatment combination for mesotheliomathe approval came on october 2 2020 and is the first approval of this kind in over 15 years.
Fda approved the novottf 100l system known as optune lua in the us as a treatment for mpm in may 2019. More than 13000 people are diagnosed with mesothelioma in europe annually. Food and drug administration fda has approved the novottf 100l system in combination with pemetrexed plus platinum based chemotherapy for the first line treatment of unresectable locally advanced or metastatic malignant pleural mesothelioma mpm.
Nvcr today announced that the us. Approved under a humanitarian device exemption novottf 100l and pemetrexed plus. Fda approved the novottf 100l system known as optune lua in the us as a treatment for mpm in may 2019 under the humanitarian device exemption hde pathway.
Food and drug administration fda has approved the novottf 100l system in combination with pemetrexed plus platinum based chemotherapy for. Nvcr today announced that the us.
More From Quartz Wool Mesothelioma
- Norfolk Mesothelioma Attorney
- Preschool Coloring Pages Butterfly
- Court Lawyer Clipart
- Ms Meyers Dish Soap
- Packard Law
Incoming Search Terms:
- 2 Packard Law,
- Fda Approves First New Treatment For Mesothelioma In 15 Years Packard Law,
- Novocure Updates To Thesis And 2019 Outlook Nasdaq Nvcr Seeking Alpha Packard Law,
- Novocure Announces Optune Lua As The Brand Name For The Novottf 100l System Packard Law,
- Acrofan Packard Law,
- Nvcr 20191231 Packard Law,