Novocure Fda Approval Mesothelioma, Could An Easy To Use Device Treat Pleural Mesothelioma
Novocure fda approval mesothelioma Indeed lately has been sought by users around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to see video and image data for inspiration, and according to the name of the article I will discuss about Novocure Fda Approval Mesothelioma.
- Novottf 100l System H180002 Fda
- Novocure Good News On All Fronts Nasdaq Nvcr Seeking Alpha
- Novocure Receives Ce Mark For Novottf 100l System Canadian Insider
- Fda Approves First New Treatment For Mesothelioma In 15 Years
- First New Mesothelioma Treatment In 15 Years Approved By Fda Medtruth Prescription Drug Medical Device Safety Informed Advocacy
- Why Novocure Stock Jumped 20 5 In May The Motley Fool
Find, Read, And Discover Novocure Fda Approval Mesothelioma, Such Us:
- Novocure Nets Approval For Its Tumor Treating Fields Therapy In Mesothelioma Fiercebiotech
- First New Mesothelioma Treatment In 15 Years Approved By Fda By Medtruth Medium
- Pleural Mesothelioma Diagnosis Target Of New Fda Approved Treatment Top Class Actions
- Novocure Receives Ce Mark For Novottf 100l System Canadian Insider
- Treatment For Malignant Pleural Mesothelioma Gets Fda Approval Cancer Therapy Advisor
- Hartford Mesothelioma Attorney
- Jacoby Ellsbury Kelsey Hawkins
- Free Printable Horse Pictures To Color
- Mark Baker Lawyer
- National Institute Of Health Immunotherapy Mesothelioma
If you re looking for National Institute Of Health Immunotherapy Mesothelioma you've come to the ideal location. We ve got 103 images about national institute of health immunotherapy mesothelioma including pictures, photos, pictures, backgrounds, and more. In these web page, we also provide variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.

Novocure Wins Fda Approval For First Mesothelioma Treatment In 15 Years Intelligence Pharma National Institute Of Health Immunotherapy Mesothelioma
If you have pleural mesothelioma and want to learn more about the novottf 100l system optune lua we can answer your questions.

National institute of health immunotherapy mesothelioma. In the first year since fda approval novocures device has checked off quite a few accomplishments. Shares of novocure are up more than 6 in premarket trading after the company announced it won regulatory approval for its mesothelioma treatment novottf 100l system. Approved under a humanitarian device exemption novottf 100l and pemetrexed plus.
Due to the clinical and pathological course of mesothelioma standard treatment methods rarely lead to a complete cure and new effective treatments are desperately needed. Novocure honors mesothelioma day with activities to raise awareness of mesothelioma and support the community september 25 2020 0400 pm eastern daylight time. Novocure based in the uk said the us.
Helier jerseybusiness wire novocure nasdaq. Novottf 100l is a non invasive antimitotic cancer treatment designed to deliver electric fields at special frequencies to disrupt solid tumor cancer cell division. Food and drug administration fda has approved the novottf 100l system in combination with pemetrexed plus platinum based chemotherapy for the first line treatment of unresectable locally advanced or metastatic malignant pleural mesothelioma mpm.
An approved hde would allow novocure to market ttfields in combination with standard of care chemotherapy as a treatment for pleural mesothelioma in the united states. Many more are still to come. Food and drug administration fda approved novottf 100l system in combination with pemetrexed plus platinum based chemotherapy as a first line treatment of unresectable locally.
Fda approved the novottf 100l system known as optune lua in the us as a treatment for mpm in may 2019. Nvcr today announced that the us. The fda has approved the novottf 100l system novocure in combination with pemetrexed plus platinum based chemotherapy for the first line treatment of unresectable locally advanced or metastatic.
More than 13000 people are diagnosed with mesothelioma in europe annually. Novocure nsdqnvcr announced that it has received fda approval for its novottf 100l system with chemotherapy for treating a type of mesothelioma.

Fda Approves Novocure S Novottf 100l System In Combination With Chemotherapy For The Treatment Of Malignant Pleural Mesothelioma National Institute Of Health Immunotherapy Mesothelioma
More From National Institute Of Health Immunotherapy Mesothelioma
- Dinosaur Pictures To Print And Colour
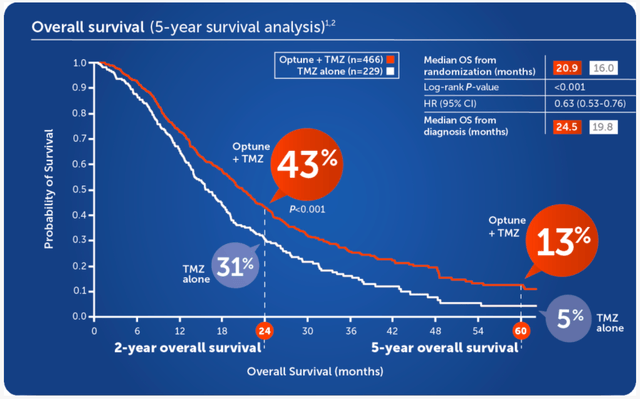
- Mesothelioma 5 Year Survival
- Dinosaur Pictures To Color Printables
- Coloring Sheet Coloring Pages To Color Online For Free For Adults
- Side Effects Of Mesothelioma Treatment
Incoming Search Terms:
- Novocure Announces Over 100 Physicians In The U S Now Certified To Prescribe Optune Lua Novocure Side Effects Of Mesothelioma Treatment,
- Https 3sj0u94bgxp33grbz1fkt62h Wpengine Netdna Ssl Com Wp Content Uploads 2019 04 Novocure 2018 Form10k Pdf Side Effects Of Mesothelioma Treatment,
- Novottf 100l For Mesothelioma Now Called Optune Lua Mesothelioma Guide Side Effects Of Mesothelioma Treatment,
- Fda Approves First New Treatment For Mesothelioma In 15 Years Side Effects Of Mesothelioma Treatment,
- Fda Approves First New Treatment For Mesothelioma In 15 Years Side Effects Of Mesothelioma Treatment,
- Fda Approves The Novottf 100ltm System In Combination With Chemotherapy For The Treatment Of Malignant Pleural Mesothelioma Business Wire Side Effects Of Mesothelioma Treatment,