Modified Recist Criteria Mesothelioma, Pdf Modified Recist Criteria For Assessment Of Response In Malignant Pleural Mesothelioma Anna Nowak Academia Edu
Modified recist criteria mesothelioma Indeed recently is being hunted by consumers around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to see video and image data for inspiration, and according to the title of the article I will discuss about Modified Recist Criteria Mesothelioma.
- Existing Models But Not Neutrophil To Lymphocyte Ratio Are Prognostic In Malignant Mesothelioma British Journal Of Cancer
- 2
- Current Therapies For Malignant Pleural Mesothelioma Environmental Health And Preventive Medicine Full Text
- Pdf How I Treat Malignant Pleural Mesothelioma
- Comparison Of Who And Recist Criteria For Response In Metastatic Colorectal Carcinoma
- Frontiers Immunotherapy In Malignant Pleural Mesothelioma Oncology
Find, Read, And Discover Modified Recist Criteria Mesothelioma, Such Us:
- Gene Therapy For Mesothelioma Us 2019 314 378 A1 Patentswarm
- Pdf Modified Recist Criteria For Assessment Of Response In Malignant Pleural Mesothelioma Semantic Scholar
- Clinical Diagnosis Of Malignant Pleural Mesothelioma Bianco Journal Of Thoracic Disease
- Revised Modified Response Evaluation Criteria In Solid Tumors For Assessment Of Response In Malignant Pleural Mesothelioma Version 1 1 Journal Of Thoracic Oncology
- 2
- Reactive Mesothelial Cells In Peritoneal Fluid
- Daniel Klein Dentist
- Mesothelioma Rare Cancer
- Harry Potter Coloring Pages Ravenclaw
- Mesothelioma Gross Specimen
If you re searching for Mesothelioma Gross Specimen you've come to the perfect location. We ve got 102 graphics about mesothelioma gross specimen adding pictures, photos, photographs, wallpapers, and more. In such webpage, we additionally have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.

Us 2011 0263512 A1 Conjugates For The Treatment Of Mesothelioma The Lens Free Open Patent And Scholarly Search Mesothelioma Gross Specimen
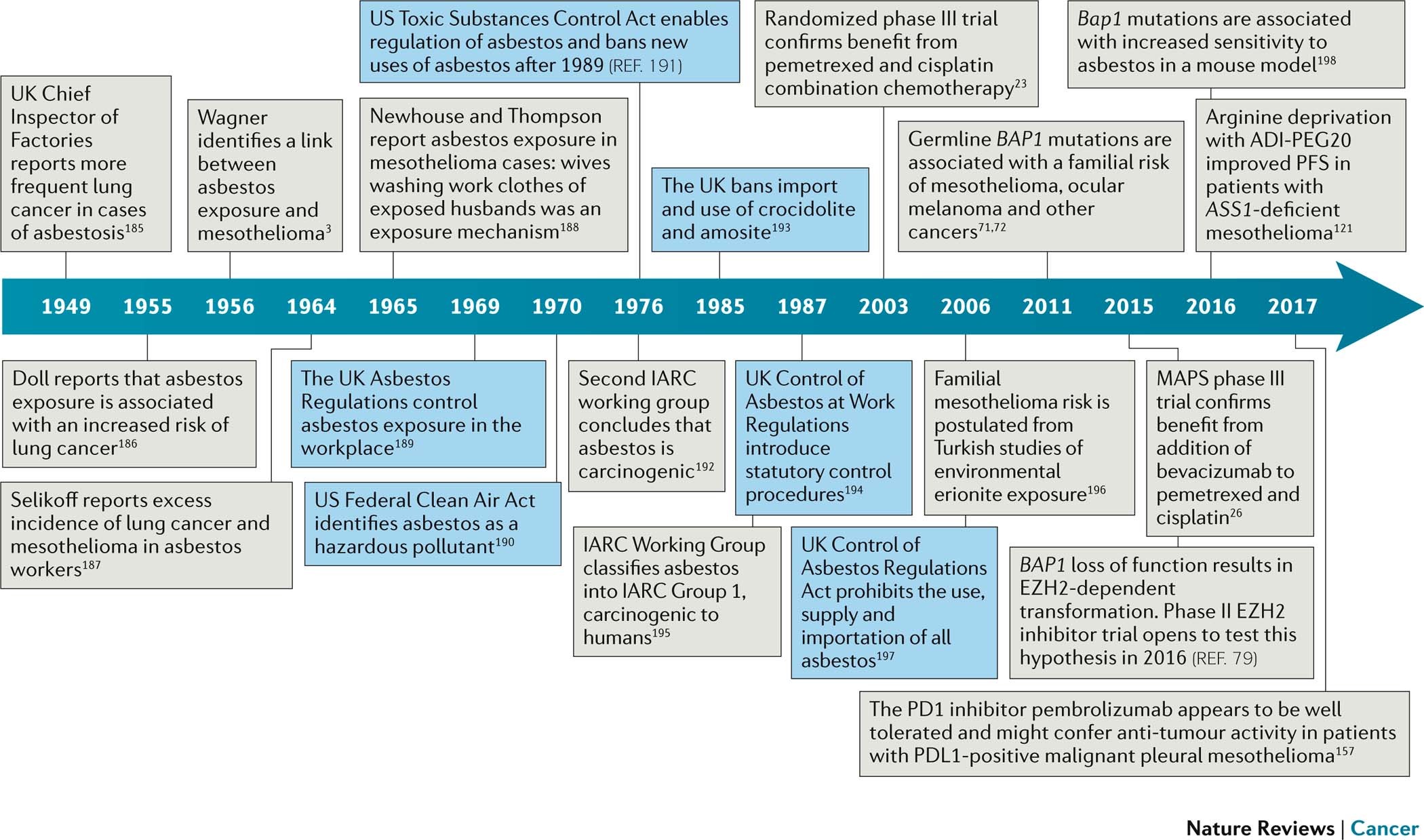
Tumour thickness perpendicular to the chest wall or mediastinum was measured in two positions at three separate levels on thoracic ct scans.

Mesothelioma gross specimen. Modified recist mrecist for mesothelioma developed to address the limitations of recist when applied to the unique morphology and growth pattern of this disease has become the standard response assessment paradigm for mesothelioma clinical trials2 3 4 5 6 mrecist specifically changed only the approach to tumor measurement from longest tumor diameter which is ill defined in mesothelioma to tumor thickness perpendicular to the chest wall or mediastinum and limited the number of. We have developed modified recist criteria that are specifically designed to address the unique growth pattern of pleural malignant mesothelioma. The current de facto standard for the assessment of mesothelioma tumor response modified recist response evaluation criteria in solid tumors was published in 2004 as a research paper.
The modified recist1 system that was published in 2004 enabled more accurate measurements of the pleural rind by implementing six perpendicular chest wall measurements from three separate. We have developed and validated modified recist criteria adapted to the growth pattern of malignant pleural mesothelioma. The application of recist criteria could however be variably interpreted by different investigators in mesothelioma and this may lead to unsatisfactory results.
We have developed modified recist criteria that are specifically designed to address the unique growth pattern of pleural malignant mesothelioma. Modified recist criteria modified recist criteria were developed. Response assessment in mesothelioma using the response evaluation criteria in solid tumors recist continues to be challenging with higher disagreement between investigators that with other solid tumors.
Use of these modified response criteria did not materially alter the response rates in our two previous trials. The current clinical method for tumor response assessment in mesothelioma is the modified response evaluation criteria in solid tumors recist guidelines which calls for two linear measurements of tumor thickness to be summed from each of three axial sections primarily in computed tomography ct scans 6 7. Practical application of the modified recist guidelines has suffered from varied interpretations resulting in inaccuracies and inconsistencies in tumor response assessment across and within mesothelioma clinical trials.
The major problems in applying the recist criteria to malignant pleural mesothelioma are in the interpreta tion of the meaning and placement of the longest unidimensional diameter of the target tumour mass to be measured. The longest diameter of a tumour mass. We evaluated 73 patients from two clinical trials of cisplatingemcitabine chemotherapy in malignant pleural mesothelioma.
More From Mesothelioma Gross Specimen
- Pumpkin Scraping Ideas
- Peritoneal Malignant Mesothelioma Radiology
- Dave Staley Mesothelioma
- Reactive Mesothelial Cells Histology
- Kramer Law
Incoming Search Terms:
- References In Tremelimumab As Second Line Or Third Line Treatment In Relapsed Malignant Mesothelioma Determine A Multicentre International Randomised Double Blind Placebo Controlled Phase 2b Trial The Lancet Oncology Kramer Law,
- Pdf How I Treat Malignant Pleural Mesothelioma Kramer Law,
- Promising Treatment Option For Unresectable Pleural Mesothelioma Iolearning Kramer Law,
- Bts Statement On Malignant Mesothelioma In The Uk Brit Thoracic Kramer Law,
- Pdf Assessment Of Tumor Response In Malignant Pleural Mesothelioma Dario Poretti Academia Edu Kramer Law,
- Questioning The Prognostic Role Of Bap 1 Immunohistochemistry In Malignant Pleural Mesothelioma A Single Center Experience With Systematic Review And Meta Analysis Lung Cancer Kramer Law,