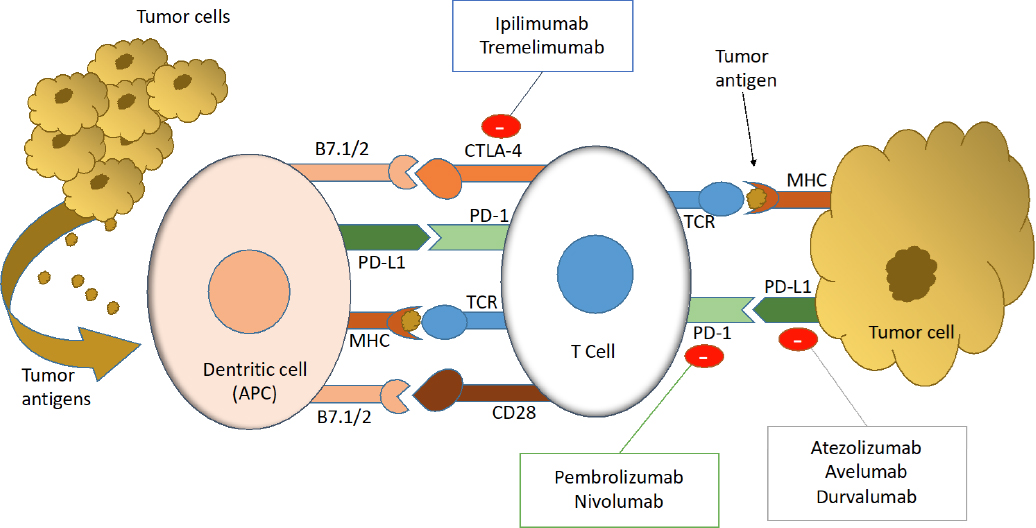
Mesothelioma Tremelimumab Approval, Tremelimumab Does Not Prolong Overall Survival In Malignant Mesothelioma Cancer Therapy Advisor
Mesothelioma tremelimumab approval Indeed recently is being sought by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to see video and image data for inspiration, and according to the title of this article I will discuss about Mesothelioma Tremelimumab Approval.
- Immunotherapy For Mesothelioma A Critical Review Of Current Clinical Trials And Future Perspectives Abstract Europe Pmc
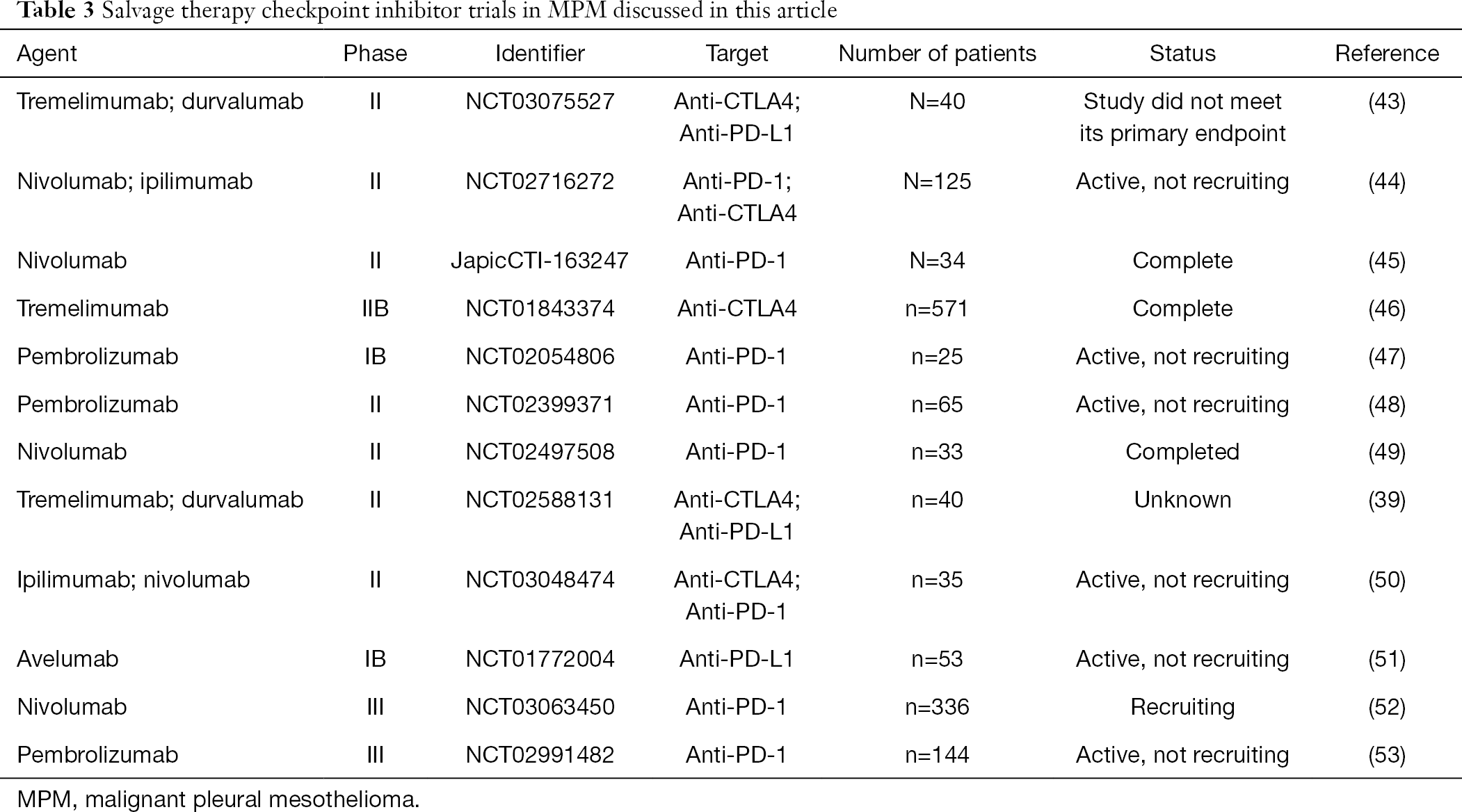
- Selected Immunotherapy Trials In Malignant Mesothelioma Download Table
- Astrazeneca S Tremelimumab Receives Fda Approval To Treat Mesothelioma
- Full Text Safety And Efficacy Of Durvalumab Medi4736 In Various Solid Tumors Dddt
- Exposure Response Analysis Of Overall Survival For Tremelimumab In Unresectable Malignant Mesothelioma The Confounding Effect Of Disease Status Baverel 2019 Clinical And Translational Science Wiley Online Library
- Immunotherapy For Mesothelioma Emerging Treatment
Find, Read, And Discover Mesothelioma Tremelimumab Approval, Such Us:
- Mesothelioma Scientific Clues For Prevention Diagnosis And Therapy Carbone 2019 Ca A Cancer Journal For Clinicians Wiley Online Library
- Durvalumab Phase Iii Trial Could Change Mesothelioma Care
- Mesothelioma Immunotherapy Types Benefits Clinical Trials
- Us Fda Approves Bristol Myers Opdivo Yervoy Combo For Another Lung Cancer Type S P Global Market Intelligence
- Nivo Lution In Mesothelioma Clinical Cancer Research
- Mesothelioma Cytology Pathology
- Pleural Thickening Causes
- Jenner Law Firm
- Simple Cat Pumpkin Carving
- Cd15 Mesothelioma
If you are searching for Cd15 Mesothelioma you've come to the perfect place. We have 100 graphics about cd15 mesothelioma adding images, pictures, photos, wallpapers, and more. In such page, we additionally have variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
Pfizer inc new york ny usa.

Cd15 mesothelioma. Each participant received at least one dose each of tremelimumab and durvalumab. It has been undergoing human trials for the treatment of various cancers but has not attained approval for any. Calabro l morra a fonsatti e et al.
Tremelimumab formerly known as cp 675206 and ticilimumab. When combined with durvalumab brand name imfinzi an fda approved immunotherapy drug for cancer patients had a better response. A new study on the immunotherapy drug tremelimumab for the treatment of mesothelioma has some good news and some bad news for mesothelioma patients and families.
Tremelimumab formerly ticilimumab cp 675206 is a fully human monoclonal antibody against ctla 4. In the companys press release robert iannone head of immuno oncology at astrazenecas global medicines development division said that there is a crucial need to develop. The uk based pharmaceutical company astrazeneca announced on wednesday that the us.
It is an immune checkpoint blockerpreviously in development by pfizer it is now in investigation by medimmune a wholly owned subsidiary of astrazeneca. Astrazenecas tremelimumab receives fda approval to treat mesothelioma. The study conducted from oct 30 2015 to oct 12 2016 consisted of 40 mesothelioma patients.
Food drug administration fda has granted tremelimumab orphan drug status for the treatment of malignant mesothelioma. Fda for its anti ctla 4 monoclonal antibody tremelimumab to treat malignant mesothelioma. And astrazeneca from 2015 is an anti ctla 4 antibody that has been studied in clinical trials for melanoma colon cancer gastric cancer and mesothelioma1012 in this review we will discuss the use of tremelimumab in all these different clinical.
An open label single arm phase 2 trial. In april 2015 tremelimumab received orphan drug designation by the fda to treat mesothelioma. Tremelimumab which has no brand name yet has not been approved by the us.
Tremelimumab for patients with chemotherapy resistant advanced malignant mesothelioma. Mesothelioma is a rare and aggressive cancer that affects the lining of the lungs and abdomen and limited treatments are available for this cancer. According to the uk authors of the new report a number of studies indicate that tremelimumab shows promise as a potential new therapy for mesotheliomaon the other hand it is still unclear whether the cost of the new drug will be.
Tremelimumab is a monoclonal antibody drug which is a type of treatment that helps the immune system fight cancer.
More From Cd15 Mesothelioma
- Wolf Costume Kids
- Simmons Beautyrest Platinum Spring Grove
- Christmas Coloring Pages Printable
- Cartoon Characters Coloring Pages
- Mesothelioma Is A Bot Wow Classic
Incoming Search Terms:
- Mesothelioma Immunotherapy Types Benefits Clinical Trials Mesothelioma Is A Bot Wow Classic,
- Us Fda Approves Bristol Myers Opdivo Yervoy Combo For Another Lung Cancer Type S P Global Market Intelligence Mesothelioma Is A Bot Wow Classic,
- Putting The Newest Mesothelioma Orphan Drug Into Perspective Mesothelioma Is A Bot Wow Classic,
- Ijms Free Full Text Combination Of Ipilimumab And Nivolumab In Cancers From Clinical Practice To Ongoing Clinical Trials Html Mesothelioma Is A Bot Wow Classic,
- Management Of Pleural Mesothelioma Thoracic Key Mesothelioma Is A Bot Wow Classic,
- Astrazeneca S Tremelimumab Receives Fda Approval To Treat Mesothelioma Mesothelioma Is A Bot Wow Classic,