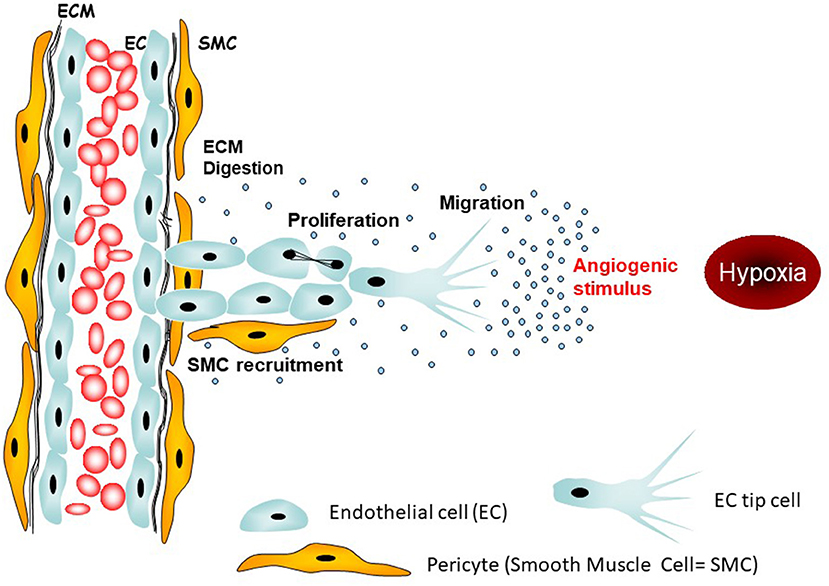
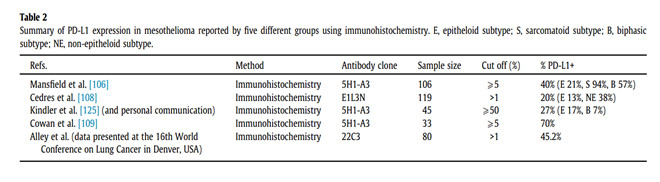
Kindler Pembrolizumab Mesothelioma, Ers Ests Eacts Estro Guidelines For The Management Of Malignant Pleural Mesothelioma European Respiratory Society
Kindler pembrolizumab mesothelioma Indeed lately has been sought by consumers around us, maybe one of you. Individuals now are accustomed to using the net in gadgets to see video and image data for inspiration, and according to the title of this article I will discuss about Kindler Pembrolizumab Mesothelioma.
- Full Text Spotlight On Bevacizumab And Its Potential In The Treatment Of Maligna Ott
- A Phase I Trial Of Surgical Resection And Intraoperative Hyperthermic Cisplatin And Gemcitabine For Pleural Mesothelioma Journal Of Thoracic Oncology
- Ers Ests Eacts Estro Guidelines For The Management Of Malignant Pleural Mesothelioma European Respiratory Society
- What Can We Offer A Patient With A Malignant Mesothelioma Iaslc
- Bevacizumab Avastin In Cancer Treatment A Review Of 15 Years Of Clinical Experience And Future Outlook Cancer Treatment Reviews
- A Systematic Review And Meta Analysis Of Second Line Therapies For Treatment Of Mesothelioma Respiratory Medicine
Find, Read, And Discover Kindler Pembrolizumab Mesothelioma, Such Us:
- Cancers Free Full Text Photodynamic Therapy Based Dendritic Cell Vaccination Suited To Treat Peritoneal Mesothelioma Html
- Pdf Immunotherapy In Malignant Pleural Mesothelioma
- Progress In The Management Of Malignant Pleural Mesothelioma In 2017 Sciencedirect
- Medical Treatment Of Malignant Pleural Mesothelioma Relapses Petrini Journal Of Thoracic Disease
- Diagnosis And Management Of Mesothelioma Liou Ame Medical Journal
- Denton St Lawrence Cricket Club
- Unicorn Mermaid Coloring Pages
- Pleurectomy Decortication Mesothelioma
- Pleural Thickening Hrct
- Peritoneal Mesothelioma Epidemiology Who
If you are searching for Peritoneal Mesothelioma Epidemiology Who you've reached the ideal location. We ve got 100 images about peritoneal mesothelioma epidemiology who adding images, photos, photographs, backgrounds, and more. In such web page, we additionally have number of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.

What Can We Offer A Patient With A Malignant Mesothelioma Iaslc Peritoneal Mesothelioma Epidemiology Who
Wclc 2016 abstract oa1302.

Peritoneal mesothelioma epidemiology who. One group received 200 mg of keytruda every three weeks. A phase ii trial by the cancer and leukemia group blungcancer. Keytruda pembrolizumab was developed by the global healthcare company merck.
The treatment is not currently available to mesothelioma sufferers through the nhs but funding for the treatment can pursued as part of a personal injury claim by those. To determine the objective response rate of patients with malignant mesothelioma treated with pembrolizumab in a an unselected patient population as well as b in a programmed cell death ligand 1 pd l1 positive population should the trial proceed to part b and pd l1 expression correlate. Zauderer mg kass sl woo k sima cs ginsberg ms krug lm.
In the new phase iii study doctors divided 144 relapsed mesothelioma patients into two groups. A new type of immunotherapy drug pembrolizumab also known by its brand name keytruda has shown significant effectiveness in the treatment of malignant pleural mesothelioma. The therapy is currently in clinical trials to test its efficacy as a mesothelioma cancer treatment.
It is an antibody drug that has been shown to improve a mesothelioma patients prognosis. Mauti la et al pembrolizumab as second or further line treatment in relapsed malignant pleural mesothelioma. Disappointing results with pembrolizumab for mesothelioma.
Pd l1 is a protein in some peoples bodies that suppresses their immune system. Esmo 2017 abstract 1615o. Kindler h et al phase ii trial of pembrolizumab in patients with malignant mesothelioma mm.
There was no treatment related mortality and no discontinuations were attributable to treatment related adverse events. P 200 mg was given q21 days. Cancer cells use this protein to keep the immune system from realizing that they are dangerous.
This phase ii trial studies how well pembrolizumab works in treating patients with malignant mesothelioma a cancer of the linings around the lungs pleura or abdomen peritoneum. Gemcitabine for malignant mesothelioma. Monoclonal antibodies such as pembrolizumab work by blocking a protein called programmed cell death 1 pd 1 which may stimulate an immune response and kill tumor cells.
We conducted a phase ii trial nct02399371 of p in previously treated mm to characterize activity in a non selected population and determine a pd l1 expression threshold methods. That trial focused on patients with relapsed cancer. Kindler hl millard f herndon je 2nd vogelzang nj suzuki y green mr.
Keytruda also known by its generic name pembrolizumab has been approved to treat non small cell lung cancer nsclc melanoma and head and neck squamous cell cancer hnscc among other cancers. Pembrolizumab appears to elicit significant clinical activity with durable responses and a manageable safety and toxicity profile in patients with pd l1 positive malignant pleural mesothelioma. Ct scans q9 weeks.

Pdf Oa13 02 Phase Ii Trial Of Pembrolizumab In Patients With Malignant Mesothelioma Mm Interim Analysis Peritoneal Mesothelioma Epidemiology Who
More From Peritoneal Mesothelioma Epidemiology Who
- Lawyer Sweeney
- Rose Coloring Pages Lol
- Mesothelioma Treatment In Dogs
- Puzzle Coloring Page
- The Semrad Law Firm
Incoming Search Terms:
- Mesothelioma Keytruda Benefits Risks More The Semrad Law Firm,
- A Multicentre Randomised Phase Iii Trial Comparing Pembrolizumab Versus Single Agent Chemotherapy For Advanced Pre Treated Malignant Pleural Mesothelioma The European Thoracic Oncology Platform Etop 9 15 Promise Meso Trial Sciencedirect The Semrad Law Firm,
- Pdf Retrospective Evaluation Of The Use Of Pembrolizumab In Malignant Mesothelioma In A Real World Australian Population The Semrad Law Firm,
- Diagnosis And Management Of Mesothelioma Liou Ame Medical Journal The Semrad Law Firm,
- What Can We Offer A Patient With A Malignant Mesothelioma Iaslc The Semrad Law Firm,
- Mesothelioma Keytruda Benefits Risks More The Semrad Law Firm,