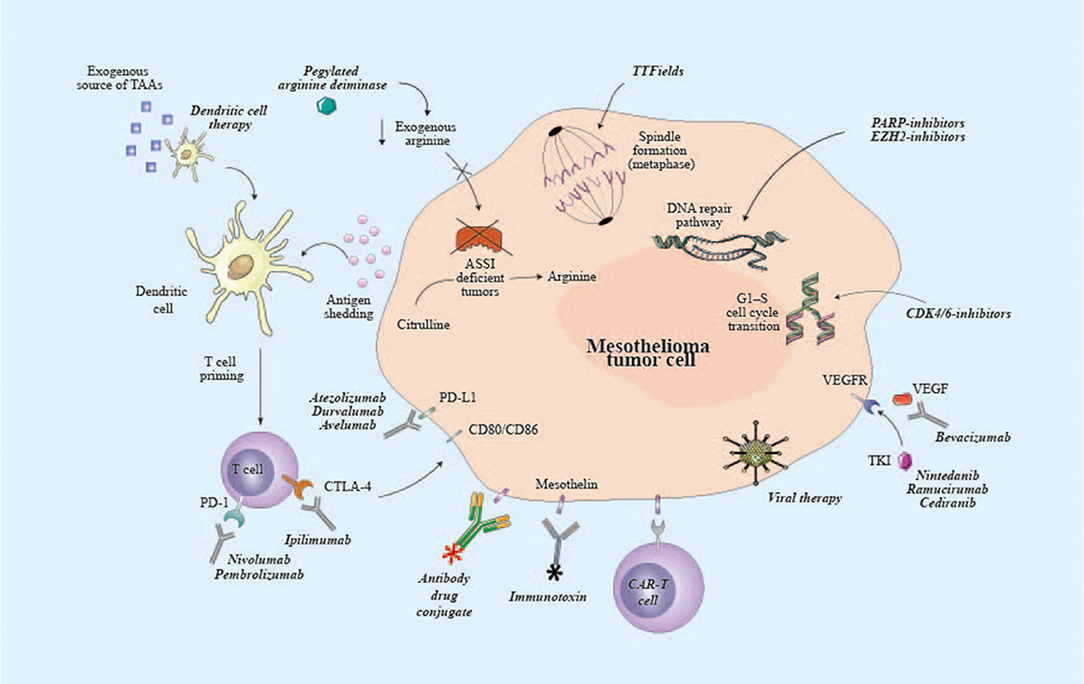
Fda Keytruda Mesothelioma, Another Win For Mesothelioma Immunotherapy Drug Keytruda
Fda keytruda mesothelioma Indeed lately is being sought by users around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to view image and video information for inspiration, and according to the title of the article I will talk about about Fda Keytruda Mesothelioma.
- Fda Approves Opdivo Yervoy Combo For Inoperable Mesothelioma
- Keytruda Approved By Fda For Further Potential Treatments Of Mesothelioma
- Fda Approves Keytruda For Certain Mesothelioma Patients
- Immunotherapy Drugs Prove Better For Treating Mesothelioma Than Chemotherapy
- Keytruda Lung Cancer And Mesothelioma Mesothelioma Com
- Mesothelioma Immunotherapy Types Benefits Clinical Trials
Find, Read, And Discover Fda Keytruda Mesothelioma, Such Us:
- Keytruda Shows Promise Treating Pleural Mesothelioma
- Two Powerhouse Immunotherapies Combined In Mesothelioma Trial
- Fda Approved Keytruda Could Help Mesothelioma Patients Mesothelioma Com
- Tremelimumab In Trial With Combination Therapy
- Pembrolizumab Medipaper Medical Communications Ltd
- Ott Law Firm
- Lady Lawyer Clipart
- Recommended Family Lawyers
- Indian Mandala Coloring Pages
- Mesothelioma Law Firm New Mexico
If you are searching for Mesothelioma Law Firm New Mexico you've come to the ideal location. We have 100 graphics about mesothelioma law firm new mexico including images, photos, pictures, backgrounds, and much more. In such web page, we also provide variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
The fda approval of keytruda marks only the second time they have approved a medication for this disease.

Mesothelioma law firm new mexico. It is still the primary chemotherapy drug for mesothelioma. Food and drug administration fda has approved mercks keytruda pembrolizumab immunotherapy drug as a treatment for advanced non small cell lung cancer nsclc recently. Regardless of how many pd l1s are in a patients body keytruda can decrease their risk of dying by 36.
Pembrolizumab often known by the brand name keytruda is a well known immunotherapy drug already being used with mixed success for several cancers. Overview how keytruda treats mesothelioma. Keytruda use in mesothelioma for patients with high mutation levels and without other options.
Keytruda is an immunotherapy drug categorized as an immune checkpoint inhibitor a type of monoclonal antibody. Food and drug administration approved the use of pembrolizumab for certain metastatic tumors this week. Approval for keytruda expands hope for mesothelioma patients.
These can show whether or not your mesothelioma is responding to treatment by making the cancer. The first mesothelioma drug was alimta. How you will know if keytruda is working.
It won fda approval in 2004. In june of 2019 the fda announced a new indication for keytruda. After being studied in more than 600 clinical trials worldwide the fda approved keytruda in 2020 for metastatic mesothelioma patients with specific genetic markers.
There are now more than ninety on going clinical trials using keytruda to treat various types of cancers. The approval was specifically provided for patients with mesothelioma tumors and other tumors that contain levels of tissue tumor mutation that are considered high as determined by an fda approved test whose disease has progressed despite having received other treatments and for whom there are no. Keytruda and immunotherapy for mesothelioma.
Patients with pleural mesothelioma cancer may have another treatment option after the us. Keytruda is the second drug the fda has approved for nsclc patients this year. Your doctor will tell you if keytruda is working after blood tests and scans like ct scans or x rays are conducted.
This date is a little more than one year after the fda granted approval for a tumor treating fields device for pleural mesothelioma. This latest fda approval is for the treatment of. Last year the fda also approved tumor treating fields now called optune lua for pleural mesothelioma.
Earlier this year the fda expanded its approval for opdivo another immunotherapy drug used to treat advanced nsclc. Despite keytrudas fda approval the survival increase is just 6 more than chemotherapy. The fdas approval occurred tuesday june 16 2020.
The drug works by blocking the pd l1 protein which is a protein that can be found on normal and cancer cells. The approval came after data from a phase 3 clinical trial demonstrated that the drug drastically improved the overall survival rate of patients with lung cancer.
Us Fda Approves Bristol Myers Opdivo Yervoy Combo For Another Lung Cancer Type S P Global Market Intelligence Mesothelioma Law Firm New Mexico
More From Mesothelioma Law Firm New Mexico
- Tree Rex Skylanders Coloring Pages
- Michael Davis Attorney
- Free Coloring Pages For Adults App
- Nail Halloween Designs Pictures
- Mesothelioma Trials Leicester
Incoming Search Terms:
- Could Tumor Treating Fields And Keytruda Work Together For Mesothelioma Mesothelioma Trials Leicester,
- Flasco Flasco Mesothelioma Trials Leicester,
- New Mesothelioma Podcast Discusses Oncos 102 And Keytruda In Clinical Trial Mesothelioma Trials Leicester,
- Keytruda Approved For Mesothelioma In Specific Cases Mesothelioma Trials Leicester,
- Keytruda Chemotherapy New Standard For Mesothelioma Mesothelioma Com Mesothelioma Trials Leicester,
- Keytruda Lawsuits Keytruda Settlements Consumer Alert Now Mesothelioma Trials Leicester,