Cisplatin Pemetrexed Bevacizumab Mesothelioma, Mesothelioma Treatment Are We On Target A Review Sciencedirect
Cisplatin pemetrexed bevacizumab mesothelioma Indeed lately has been hunted by consumers around us, maybe one of you. People now are accustomed to using the net in gadgets to see image and video information for inspiration, and according to the title of the article I will discuss about Cisplatin Pemetrexed Bevacizumab Mesothelioma.
- Malignant Pleural Mesothelioma Updates
- Full Text Spotlight On Bevacizumab And Its Potential In The Treatment Of Maligna Ott
- Ijms Free Full Text Repurposing Quinacrine For Treatment Of Malignant Mesothelioma In Vitro Therapeutic And Mechanistic Evaluation Html
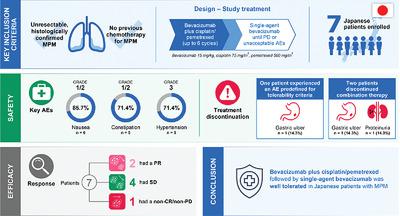
- Bevacizumab Plus Cisplatin Pemetrexed Then Bevacizumab Alone For Unresectable Malignant Pleural Mesothelioma A Japanese Safety Study Nakano Asia Pacific Journal Of Clinical Oncology Wiley Online Library
- Bevacizumab For Newly Diagnosed Pleural Mesothelioma In The Mesothelioma Avastin Cisplatin Pemetrexed Study Maps A Randomised Controlled Open Label Phase 3 Trial The Lancet
- The Effectiveness And Safety Of Platinum Based Pemetrexed And Platinum Based Gemcitabine Treatment In Patients With Malignant Pleural Mesothelioma Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For
Find, Read, And Discover Cisplatin Pemetrexed Bevacizumab Mesothelioma, Such Us:
- Standard Mesothelioma Chemotherapy With Avastin More Good News
- Full Text Spotlight On Bevacizumab And Its Potential In The Treatment Of Maligna Ott
- Shorter Survival In Malignant Pleural Mesothelioma Patients With High Pd L1 Expression Associated With Sarcomatoid Or Biphasic Histology Subtype A Series Of 214 Cases From The Bio Maps Cohort Clinical Lung Cancer
- Expanding Treatment Choices In Mesothelioma Exploring The Promise Of
- Pdf Multicenter Phase Ii Study On Cisplatin Pemetrexed And Bevacizumab Followed By Maintenance With Pemetrexed And Bevacizumab For Patients With Advanced Or Recurrent Nonsquamous Non Small Cell Lung Cancer Map Study
- Show Pictures Of Zombies
- What Is Asbestos Siding Look Like
- World Health Organisation Mesothelioma
- Coloring Sheet Letter C Coloring Pages
- Lymphedema Mesothelioma
If you re searching for Lymphedema Mesothelioma you've arrived at the right location. We have 100 graphics about lymphedema mesothelioma adding pictures, photos, pictures, wallpapers, and much more. In such webpage, we also have number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.

Health Related Quality Of Life In Patients With Advanced Nonsquamous Non Small Cell Lung Cancer Receiving Bevacizumab Or Bevacizumab Plus Pemetrexed Maintenance Therapy In Avaperl Mo22089 Journal Of Thoracic Oncology Lymphedema Mesothelioma
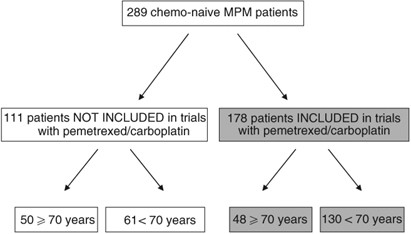
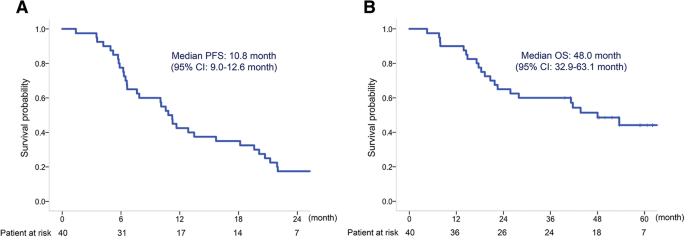
The phase 23 mesothelioma avastin cisplatin pemetrexed study maps was initiated to assess the e ect on survival of bevacizumab when added to the present standard of care cisplatin plus pemetrexed as rst line treatment of advanced malignant pleural meso thelioma.

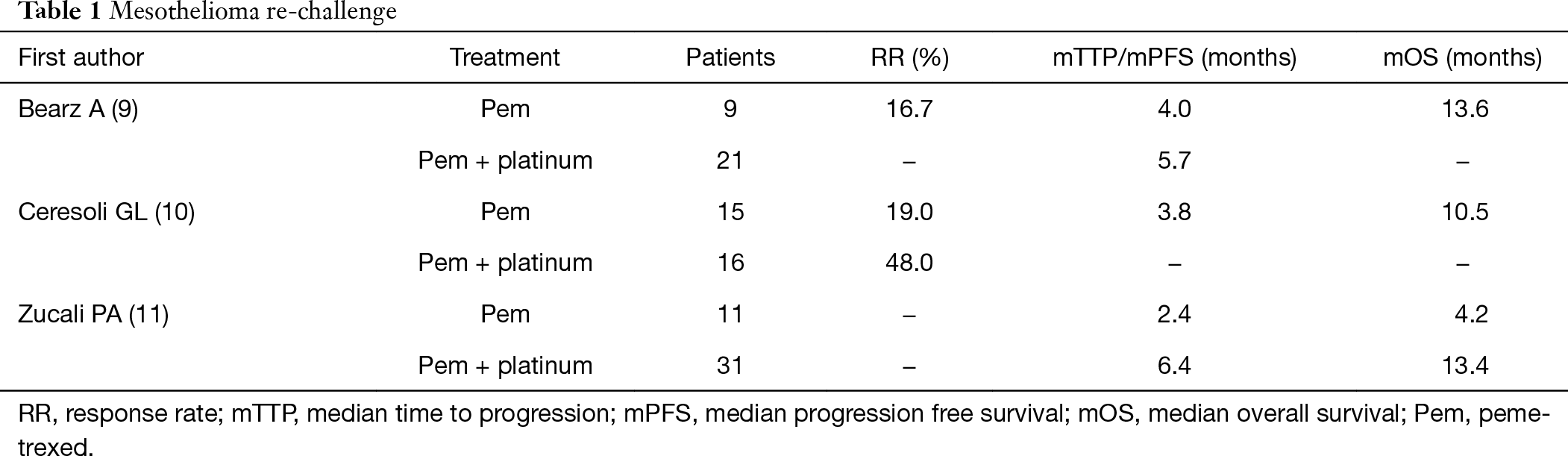
Lymphedema mesothelioma. Addition of bevacizumab to pemetrexed plus cisplatin significantly improved os in malignant pleural mesothelioma at the cost of expected manageable toxic effects therefore it should be considered as a suitable treatment for the disease. The primary phase 2 outcome was disease. Pemetrexed and cisplatin is a combination treatment used to treat mesothelioma and non small cell lung cancerit is best to read this information with our general information about chemotherapy and the type of cancer you have.
A randomised controlled open label phase 3 trial. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study maps. Epub 2015 dec 21.
A multicenter phase ii study of cisplatin pemetrexed and bevacizumab in patients with advanced malignant mesothelioma. 1division of hematologyoncology university of texas southwestern medical center dallas tx 75390 8852 usa. Your doctor will talk to you about this treatment and its possible side effects before you agree to have treatment.
Intergroupe francophone de cancerologie thoracique ifct. The primary phase 2 outcome was disease control rate at 6 months in the pcb. The phase ii.
Dowell je1 dunphy fr taub rn gerber de ngov l yan j xie y kindler hl. Bevacizumab a monoclonal anti vegf antibody was a rational approach to be tested in mpm. Addition of bevacizumab to cisplatin and pemetrexed significantly increased os and pfs in malignant pleural mesothelioma with expected and manageable toxic effects this result has important implications for future first line treatment of the disease because the pemetrexed plus cisplatin plus bevacizumab regimen should be considered as a new.
Based on the results of the phase iii ifct 0701 mesothelioma avastin cisplatin pemetrexed study cisplatin pemetrexed bevacizumab is now the accepted standard in france.
More From Lymphedema Mesothelioma
- Cool Disney Pumpkin Carvings
- Sass Law Firm
- Dilaudid Pump Mesothelioma
- Social Security Disability Attorney Near Me
- Free Curse Word Coloring Pages
Incoming Search Terms:
- Tumour Treating Fields In Combination With Pemetrexed And Cisplatin Or Carboplatin As First Line Treatment For Unresectable Malignant Pleural Mesothelioma Stellar A Multicentre Single Arm Phase 2 Trial Lancet Oncol X Mol Free Curse Word Coloring Pages,
- Pdf Bevacizumab For Newly Diagnosed Pleural Mesothelioma In The Mesothelioma Avastin Cisplatin Pemetrexed Study Maps A Randomised Controlled Open Label Phase 3 Trial Semantic Scholar Free Curse Word Coloring Pages,
- Bevacizumab For Newly Diagnosed Pleural Mesothelioma In The Mesothelioma Avastin Cisplatin Pemetrexed Study Maps A Randomised Controlled Open Label Phase 3 Trial The Lancet Free Curse Word Coloring Pages,
- Available On Line In The Bibliographic Review Section The New Paper Nintedanib New Treatment For Malignant Pleural Mesothelioma Available On Line In The Bibliographic Review Section The New Paper Nintedanib New Free Curse Word Coloring Pages,
- Expansion Phase 1 Study Of Pegargiminase Plus Pemetrexed And Cisplatin In Patients With Argininosuccinate Synthetase 1 Deficient Mesothelioma Safety Efficacy And Resistance Mechanisms Jto Clinical And Research Reports Free Curse Word Coloring Pages,
- How I Treat Malignant Pleural Mesothelioma Esmo Open Free Curse Word Coloring Pages,